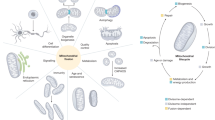
Key Points
-
Classical dynamins belong to a superfamily that includes dynamin-like proteins, optic atrophy 1 (OPA1), mitofusin and Mx proteins.
-
On the basis of sequence homology, domain structure and biological functions, we define the superfamily members from yeast and plants to man.
-
It is proposed that all members of the superfamily are membrane-binding and oligomerization-dependent GTPases.
-
On the basis of structural and biochemical comparison, it is possible to test the functions of all members of this superfamily with the use of site-directed mutagenesis.
-
It is proposed that all dynamin-superfamily members are mechanochemical GTPases that either function in membrane scission or tubulation.
-
The mechanochemical mechanism of dynamin is applied to all known biological functions of the superfamily: vesicle scission, mitochondrial fission and fusion, cytokinesis and antiviral activity.
Abstract
Dynamins are large GTPases that belong to a protein superfamily that, in eukaryotic cells, includes classical dynamins, dynamin-like proteins, OPA1, Mx proteins, mitofusins and guanylate-binding proteins/atlastins. They are involved in many processes including budding of transport vesicles, division of organelles, cytokinesis and pathogen resistance. With sequenced genomes from Homo sapiens, Drosophila melanogaster, Caenorhabditis elegans, yeast species and Arabidopsis thaliana, we now have a complete picture of the members of the dynamin superfamily from different organisms. Here, we review the superfamily of dynamins and their related proteins, and propose that a common mechanism leading to membrane tubulation and/or fission could encompass their many varied functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Grigliatti, T. A., Hall, L., Rosenbluth, R. & Suzuki, D. T. Temperature-sensitive mutations in Drosophila melanogaster. XIV. A selection of immobile adults. Mol. Gen. Genet. 120, 107–114 (1973). Identifies the Shibire, Stoned and Paralytic temperature-sensitive loci. The authors also found the Rapid Exhaustion, Bang Sensitive, and Wobbly non-temperature-sensitive loci.
Suzuki, D. T., Grigliatti, T. & Williamson, R. Temperature-sensitive mutations in Drosophila melanogaster. VII. A mutation (para-ts) causing reversible adult paralysis. Proc. Natl Acad. Sci. USA 68, 890–893 (1971).
van der Bliek, A. M. & Meyerowitz, E. M. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature 351, 411–414 (1991).
Chen, M. S. et al. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature 351, 583–586 (1991).
Shpetner, H. S. & Vallee, R. B. Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules. Cell 59, 421–432 (1989).
Obar, R. A., Collins, C. A., Hammarback, J. A., Shpetner, H. S. & Vallee, R. B. Molecular cloning of the microtubule-associated mechanochemical enzyme dynamin reveals homology with a new family of GTP-binding proteins. Nature 347, 256–261 (1990). The first cloning of dynamin showing that it was part of a superfamily of GTPases.
Robinson, P. J., Hauptschein, R., Lovenberg, W. & Dunkley, P. R. Dephosphorylation of synaptosomal proteins P96 and P139 is regulated by both depolarization and calcium, but not by a rise in cytosolic calcium alone. J. Neurochem. 48, 187–195 (1987).
Cao, H., Garcia, F. & McNiven, M. A. Differential distribution of dynamin isoforms in mammalian cells. Mol. Biol. Cell 9, 2595–2609 (1998).
Nakata, T., Takemura, R. & Hirokawa, N. A novel member of the dynamin family of GTP-binding proteins is expressed specifically in the testis. J. Cell Sci. 105, 1–5 (1993).
Sontag, J. M. et al. Differential expression and regulation of multiple dynamins. J. Biol. Chem. 269, 4547–4554 (1994).
Gray, N. W. et al. Dynamin 3 is a component of the postsynapse, where it interacts with mGluR5 and Homer. Curr. Biol. 13, 510–515 (2003).
Henley, J. R., Krueger, E. W., Oswald, B. J. & McNiven, M. A. Dynamin-mediated internalization of caveolae. J. Cell Biol. 141, 85–99 (1998).
Oh, P., McIntosh, D. P. & Schnitzer, J. E. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J. Cell Biol. 141, 101–114 (1998). References 12 and 13 are the first clear demonstrations of the involvement of dynamin in vesicle-scission events other than clathrin-mediated vesicle budding. Dynamin has now been implicated in many other budding events.
Gold, E. S. et al. Dynamin 2 is required for phagocytosis in macrophages. J. Exp. Med. 190, 1849–1856 (1999).
Ochoa, G. C. et al. A functional link between dynamin and the actin cytoskeleton at podosomes. J. Cell Biol. 150, 377–389 (2000).
van Dam, E. M. & Stoorvogel, W. Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles. Mol. Biol. Cell 13, 169–182 (2002).
Orth, J. D. & McNiven, M. A. Dynamin at the actin-membrane interface. Curr. Opin. Cell Biol. 15, 31–39 (2003).
Gammie, A. E., Kurihara, L. J., Vallee, R. B. & Rose, M. D. DNM1, a dynamin-related gene, participates in endosomal trafficking in yeast. J. Cell Biol. 130, 553–566 (1995).
Shin, H. W., Shinotsuka, C., Torii, S., Murakami, K. & Nakayama, K. Identification and subcellular localization of a novel mammalian dynamin-related protein homologous to yeast Vps1p and Dnm1p. J. Biochem. 122, 525–530 (1997).
Kamimoto, T. et al. Dymple, a novel dynamin-like high molecular weight GTPase lacking a proline-rich carboxyl-terminal domain in mammalian cells. J. Biol. Chem. 273, 1044–1051 (1998).
Hong, Y. R., Chen, C. H., Cheng, D. S., Howng, S. L. & Chow, C. C. Human dynamin-like protein interacts with the glycogen synthase kinase 3β. Biochem. Biophys. Res. Commun. 249, 697–703 (1998).
Smirnova, E., Shurland, D. L., Ryazantsev, S. N. & van der Bliek, A. M. A human dynamin-related protein controls the distribution of mitochondria. J. Cell Biol. 143, 351–358 (1998).
Imoto, M., Tachibana, I. & Urrutia, R. Identification and functional characterization of a novel human protein highly related to the yeast dynamin-like GTPase Vps1p. J. Cell Sci. 111, 1341–1349 (1998).
Labrousse, A. M., Zappaterra, M. D., Rube, D. A. & van der Bliek, A. M. C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol. Cell 4, 815–826 (1999). This is a clear demonstration of the function of dynamin-like proteins in mitochondrial division in live animals.
Wienke, D. C., Knetsch, M. L., Neuhaus, E. M., Reedy, M. C. & Manstein, D. J. Disruption of a dynamin homologue affects endocytosis, organelle morphology, and cytokinesis in Dictyostelium discoideum. Mol. Biol. Cell 10, 225–243 (1999).
Shin, H. W. et al. Intermolecular and interdomain interactions of a dynamin-related GTP-binding protein, Dnm1p/Vps1p-like protein. J. Biol. Chem. 274, 2780–2785 (1999).
Smirnova, E., Griparic, L., Shurland, D. L. & van der Bliek, A. M. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell 12, 2245–2256 (2001).
Yoon, Y., Pitts, K. R. & McNiven, M. A. Mammalian dynamin-like protein DLP1 tubulates membranes. Mol. Biol. Cell 12, 2894–2905 (2001).
Klockow, B. et al. The dynamin A ring complex: molecular organization and nucleotide-dependent conformational changes. EMBO J. 21, 240–250 (2002).
Kim, Y. W. et al. Arabidopsis dynamin-like 2 that binds specifically to phosphatidylinositol-4-phosphate assembles into a high-molecular weight complex in vivo and in vitro. Plant Physiol. 127, 1243–1255 (2001).
Rothman, J. H., Raymond, C. K., Gilbert, T., O'Hara, P. J. & Stevens, T. H. A putative GTP binding protein homologous to interferon-inducible Mx proteins performs an essential function in yeast protein sorting. Cell 61, 1063–1074 (1990).
Wilsbach, K. & Payne, G. S. Vps1p, a member of the dynamin GTPase family, is necessary for Golgi membrane protein retention in Saccharomyces cerevisiae. EMBO J. 12, 3049–3059 (1993).
Staeheli, P., Haller, O., Boll, W., Lindenmann, J. & Weissmann, C. Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to influenza virus. Cell 44, 147–158 (1986). A direct demonstation of the antiviral effect of MxA.
Janzen, C., Kochs, G. & Haller, O. A monomeric GTPase-negative MxA mutant with antiviral activity. J. Virol. 74, 8202–8206 (2000).
Kochs, G., Haener, M., Aebi, U. & Haller, O. Self-assembly of human MxA GTPase into highly ordered dynamin-like oligomers. J. Biol. Chem. 277, 14172–14176 (2002).
Gao, H., Kadirjan-Kalbach, D., Froehlich, J. E. & Osteryoung, K. W. ARC5, a cytosolic dynamin-like protein from plants, is part of the chloroplast division machinery. Proc. Natl Acad. Sci. USA 100, 4328–4333 (2003).
Miyagishima, S. Y. et al. A plant-specific dynamin-related protein forms a ring at the chloroplast division site. Plant Cell 15, 655–665 (2003). Uses a very interesting model organism, which has one mitochondrion and one chloroplast. Also shows a ring of dynamin constricting during organelle division.
Satoh, M., Hamamoto, T., Seo, N., Kagawa, Y. & Endo, H. Differential sublocalization of the dynamin-related protein OPA1 isoforms in mitochondria. Biochem. Biophys. Res. Commun. 300, 482–493 (2003).
Olichon, A. et al. The human dynamin-related protein OPA1 is anchored to the mitochondrial inner membrane facing the inter-membrane space. FEBS Lett. 523, 171–176 (2002).
Jones, B. A. & Fangman, W. L. Mitochondrial DNA maintenance in yeast requires a protein containing a region related to the GTP-binding domain of dynamin. Genes Dev. 6, 380–389 (1992).
Wong, E. D. et al. The intramitochondrial dynamin-related GTPase, Mgm1p, is a component of a protein complex that mediates mitochondrial fusion. J. Cell Biol. 160, 303–311 (2003).
Hales, K. G. & Fuller, M. T. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell 90, 121–129 (1997).
Hermann, G. J. et al. Mitochondrial fusion in yeast requires the transmembrane GTPase Fzo1p. J. Cell Biol. 143, 359–373 (1998).
Rapaport, D., Brunner, M., Neupert, W. & Westermann, B. Fzo1p is a mitochondrial outer membrane protein essential for the biogenesis of functional mitochondria in Saccharomyces cerevisiae. J. Biol. Chem. 273, 20150–20155 (1998).
Santel, A. & Fuller, M. T. Control of mitochondrial morphology by a human mitofusin. J. Cell Sci. 114, 867–874 (2001).
Prakash, B., Praefcke, G. J. K., Renault, L., Wittinghofer, A. & Herrmann, C. Structure of human guanylate-binding protein 1 representing a unique class of GTP-binding proteins. Nature 403, 567–571 (2000).
Prakash, B., Renault, L., Praefcke, G. J., Herrmann, C. & Wittinghofer, A. Triphosphate structure of guanylate-binding protein 1 and implications for nucleotide binding and GTPase mechanism. EMBO J. 19, 4555–4564 (2000).
Staeheli, P., Horisberger, M. A. & Haller, O. Mx-dependent resistance to influenza viruses is induced by mouse interferons α and β but not γ. Virology 132, 456–461 (1984).
Anderson, S. L., Carton, J. M., Lou, J., Xing, L. & Rubin, B. Y. Interferon-induced guanylate binding protein-1 (GBP-1) mediates an antiviral effect against vesicular stomatitis virus and encephalomyocarditis virus. Virology 256, 8–14 (1999).
Schwemmle, M., Kaspers, B., Irion, A., Staeheli, P. & Schultz, U. Chicken guanylate-binding protein. Conservation of GTPase activity and induction by cytokines. J. Biol. Chem. 271, 10304–10308 (1996).
Zhao, X. et al. Mutations in a newly identified GTPase gene cause autosomal dominant hereditary spastic paraplegia. Nature Genet. 29, 326–331 (2001).
Zhu, P. P. et al. Cellular localization, oligomerization, and membrane association of the hereditary Spastic Paraplegia 3A (SPG3A) protein atlastin. J. Biol. Chem. 278, 49063–49071 (2003).
Uthaiah, R. C., Praefcke, G. J., Howard, J. C. & Herrmann, C. IIGP1, an interferon-γ-inducible 47-kDa GTPase of the mouse, showing cooperative enzymatic activity and GTP-dependent multimerization. J. Biol. Chem. 278, 29336–29343 (2003).
Weber, T. et al. SNAREpins: minimal machinery for membrane fusion. Cell 92, 759–772 (1998).
Pai, E. F. et al. Refined crystal structure of the triphosphate conformation of H-ras p21 at 1.35 Å resolution: implications for the mechanism of GTP hydrolysis. EMBO J. 9, 2351–2359 (1990).
Vetter, I. R. & Wittinghofer, A. The guanine nucleotide-binding switch in three dimensions. Science 294, 1299–1304 (2001).
Niemann, H. H., Knetsch, M. L., Scherer, A., Manstein, D. J. & Kull, F. J. Crystal structure of a dynamin GTPase domain in both nucleotide-free and GDP-bound forms. EMBO J. 20, 5813–5821 (2001). The first high-resolution structure of the GTPase domain of a close homologue to dynamin 1.
Krishnan, K. S. et al. Nucleoside diphosphate kinase, a source of GTP, is required for dynamin-dependent synaptic vesicle recycling. Neuron 30, 197–210 (2001).
Shpetner, H. S. & Vallee, R. B. Dynamin is a GTPase stimulated to high levels of activity by microtubules. Nature 355, 733–735 (1992).
Tuma, P. L. & Collins, C. A. Activation of dynamin GTPase is a result of positive cooperativity. J. Biol. Chem. 269, 30842–30847 (1994). Assembly of dynamin on microtubules or acidic liposomes results in a positive cooperativity of GTP hydrolysis. This study implied that dynamin stimulates its own GTP hydrolysis on assembly.
Warnock, D. E., Hinshaw, J. E. & Schmid, S. L. Dynamin self-assembly stimulates its GTPase activity. J. Biol. Chem. 271, 22310–22314 (1996).
Sever, S., Muhlberg, A. B. & Schmid, S. L. Impairment of dynamin's GAP domain stimulates receptor-mediated endocytosis. Nature 398, 481–486 (1999). A study in which the authors suggest an alternative mechanism for dynamin as a regulatory GTPase.
Stowell, M. H., Marks, B., Wigge, P. & McMahon, H. T. Nucleotide-dependent conformational changes in dynamin: evidence for a mechanochemical molecular spring. Nature Cell Biol. 1, 27–32 (1999). A demonstration of the mechanochemical properties of dynamin, in which the authors suggest a spring or 'poppase' mechanism for vesicle scission.
Muhlberg, A. B., Warnock, D. E. & Schmid, S. L. Domain structure and intramolecular regulation of dynamin GTPase. EMBO J. 16, 6676–6683 (1997).
Smirnova, E., Shurland, D. L., Newman-Smith, E. D., Pishvaee, B. & van der Bliek, A. M. A model for dynamin self-assembly based on binding between three different protein domains. J. Biol. Chem. 274, 14942–14947 (1999).
Okamoto, P. M., Tripet, B., Litowski, J., Hodges, R. S. & Vallee, R. B. Multiple distinct coiled-coils are involved in dynamin self-assembly. J. Biol. Chem. 274, 10277–10286 (1999).
Zhang, P. & Hinshaw, J. E. Three-dimensional reconstruction of dynamin in the constricted state. Nature Cell Biol. 3, 922–926 (2001).
Hinshaw, J. E. & Schmid, S. L. Dynamin self-assembles into rings suggesting a mechanism for coated vesicle budding. Nature 374, 190–192 (1995).
Sweitzer, S. M. & Hinshaw, J. E. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell 93, 1021–1029 (1998). A demonstration of the mechanochemical properties of dynamin, in which the authors suggest a 'pinchase' mechanism of vesicle scission.
Gu, X. & Verma, D. P. Phragmoplastin, a dynamin-like protein associated with cell plate formation in plants. EMBO J. 15, 695–704 (1996). The first dynamin identified in plants.
Zhang, Z., Hong, Z. & Verma, D. P. Phragmoplastin polymerizes into spiral coiled structures via intermolecular interaction of two self-assembly domains. J. Biol. Chem. 275, 8779–8784 (2000).
Melen, K. et al. Interferon-induced Mx proteins form oligomers and contain a putative leucine zipper. J. Biol. Chem. 267, 25898–25907 (1992). The first demonstration of the oligomerization of a dynamin-superfamily member.
Nakayama, M. et al. Structure of mouse Mx1 protein. Molecular assembly and GTP-dependent conformational change. J. Biol. Chem. 268, 15033–15038 (1993).
Schumacher, B. & Staeheli, P. Domains mediating intramolecular folding and oligomerization of MxA GTPase. J. Biol. Chem. 273, 28365–28370 (1998).
Accola, M. A., Huang, B., Al Masri, A. & McNiven, M. A. The antiviral dynamin family member, MxA, tubulates lipids and localizes to the smooth endoplasmic reticulum. J. Biol. Chem. 277, 21829–21835 (2002).
Klein, D. E., Lee, A., Frank, D. W., Marks, M. S. & Lemmon, M. A. The pleckstrin homology domains of dynamin isoforms require oligomerization for high affinity phosphoinositide binding. J. Biol. Chem. 273, 27725–27733 (1998).
Lemmon, M. A. & Ferguson, K. M. Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem. J. 350, 1–18 (2000).
Vallis, Y., Wigge, P., Marks, B., Evans, P. R. & McMahon, H. T. Importance of the pleckstrin homology domain of dynamin in clathrin-mediated endocytosis. Curr. Biol. 9, 257–260 (1999).
Lee, A., Frank, D. W., Marks, M. S. & Lemmon, M. A. Dominant-negative inhibition of receptor-mediated endocytosis by a dynamin-1 mutant with a defective pleckstrin homology domain. Curr. Biol. 9, 261–264 (1999).
Achiriloaie, M., Barylko, B. & Albanesi, J. P. Essential role of the dynamin pleckstrin homology domain in receptor-mediated endocytosis. Mol. Cell Biol. 19, 1410–1415 (1999).
McQuibban, G. A., Saurya, S. & Freeman, M. Mitochondrial membrane remodelling regulated by a conserved rhomboid protease. Nature 423, 537–541 (2003). The cleavage of Mgm1 by a rhomboid protease in the inner mitochondrial membrane is essential for its activity in mitochondrial fusion.
Rojo, M., Legros, F., Chateau, D. & Lombes, A. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J. Cell Sci. 115, 1663–1674 (2002).
Cheng, Y. S., Patterson, C. E. & Staeheli, P. Interferon-induced guanylate-binding proteins lack an N(T)KXD consensus motif and bind GMP in addition to GDP and GTP. Mol. Cell Biol. 11, 4717–4725 (1991).
Gorbacheva, V. Y., Lindner, D., Sen, G. C. & Vestal, D. J. The interferon (IFN)-induced GTPase, mGBP-2. Role in IFN-γ-induced murine fibroblast proliferation. J. Biol. Chem. 277, 6080–6087 (2002).
Scheffzek, K. et al. The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science 277, 333–338 (1997).
Sever, S., Damke, H. & Schmid, S. L. Dynamin:GTP controls the formation of constricted coated pits, the rate limiting step in clathrin-mediated endocytosis. J. Cell Biol. 150, 1137–1148 (2000).
Marks, B. et al. GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature 410, 231–235 (2001). Hydrolysis of GTP by dynamin is required for vesicle scission, whereas GTP binding leads to tubulation in vivo.
Eccleston, J. F., Binns, D. D., Davis, C. T., Albanesi, J. P. & Jameson, D. M. Oligomerization and kinetic mechanism of the dynamin GTPase. Eur. Biophys. J. 31, 275–282 (2002).
Newmyer, S. L., Christensen, A. & Sever, S. Auxilin-dynamin interactions link the uncoating ATPase chaperone machinery with vesicle formation. Dev. Cell 4, 929–940 (2003).
Pitts, K. R., Yoon, Y., Krueger, E. W. & McNiven, M. A. The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol. Biol. Cell 10, 4403–4417 (1999).
Otsuga, D. et al. The dynamin-related GTPase, Dnm1p, controls mitochondrial morphology in yeast. J. Cell Biol. 143, 333–349 (1998).
Fukushima, N. H., Brisch, E., Keegan, B. R., Bleazard, W. & Shaw, J. M. The GTPase effector domain sequence of the Dnm1p GTPase regulates self-assembly and controls a rate-limiting step in mitochondrial fission. Mol. Biol. Cell 12, 2756–2766 (2001).
Kang, S. G. et al. Molecular cloning of an Arabidopsis cDNA encoding a dynamin-like protein that is localized to plastids. Plant Mol. Biol. 38, 437–447 (1998).
Park, J. M. et al. A dynamin-like protein in Arabidopsis thaliana is involved in biogenesis of thylakoid membranes. EMBO J. 17, 859–867 (1998).
Hoepfner, D., van den Berg, M., Philippsen, P., Tabak, H. F. & Hettema, E. H. A role for Vps1p, actin, and the Myo2p motor in peroxisome abundance and inheritance in Saccharomyces cerevisiae. J. Cell Biol. 155, 979–990 (2001).
Koch, A. et al. Dynamin-like protein 1 is involved in peroxisomal fission. J. Biol. Chem. 278, 8597–8605 (2003).
Li, X. & Gould, S. J. The dynamin-like GTPase Dlp1 is essential for peroxisome division and is recruited to peroxisomes in part by Pex11. J. Biol. Chem. 278, 17012–17020 (2003).
Herlan, M., Vogel, F., Bornhovd, C., Neupert, W. & Reichert, A. S. Processing of Mgm1 by the Rhomboid-type protease Pcp1 is required for maintenance of mitochondrial morphology and of mitochondrial DNA. J. Biol. Chem. 278, 27781–27788 (2003).
Wong, E. D. et al. The dynamin-related GTPase, Mgm1p, is an intermembrane space protein required for maintenance of fusion competent mitochondria. J. Cell Biol. 151, 341–352 (2000).
Sesaki, H. & Jensen, R. E. Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. J. Cell Biol. 147, 699–706 (1999).
Lukowitz, W., Mayer, U. & Jurgens, G. Cytokinesis in the Arabidopsis embryo involves the syntaxin-related KNOLLE gene product. Cell 84, 61–71 (1996).
Samuels, A. L., Giddings, T. H., Jr. & Staehelin, L. A. Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. J. Cell Biol. 130, 1345–1357 (1995).
Gu, X. & Verma, D. P. Dynamics of phragmoplastin in living cells during cell plate formation and uncoupling of cell elongation from the plane of cell division. Plant Cell 9, 157–169 (1997).
Verma, D. P. Cytokinesis and building of the cell plate in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 751–784 (2001).
Otegui, M. S., Mastronarde, D. N., Kang, B. H., Bednarek, S. Y. & Staehelin, L. A. Three-dimensional analysis of syncytial-type cell plates during endosperm cellularization visualized by high resolution electron tomography. Plant Cell 13, 2033–2051 (2001).
Feng, B., Schwarz, H. & Jesuthasan, S. Furrow-specific endocytosis during cytokinesis of zebrafish blastomeres. Exp. Cell Res. 279, 14–20 (2002).
Thompson, H. M., Skop, A. R., Euteneuer, U., Meyer, B. J. & McNiven, M. A. The large GTPase dynamin associates with the spindle midzone and is required for cytokinesis. Curr. Biol. 12, 2111–2117 (2002).
Haller, O. & Kochs, G. Interferon-induced mx proteins: dynamin-like GTPases with antiviral activity. Traffic 3, 710–717 (2002).
Kochs, G. & Haller, O. Interferon-induced human MxA GTPase blocks nuclear import of Thogoto virus nucleocapsids. Proc. Natl Acad. Sci. USA 96, 2082–2086 (1999).
Kochs, G. & Haller, O. GTP-bound human MxA protein interacts with the nucleocapsids of Thogoto virus (Orthomyxoviridae). J. Biol. Chem. 274, 4370–4376 (1999).
Guenzi, E. et al. The helical domain of GBP-1 mediates the inhibition of endothelial cell proliferation by inflammatory cytokines. EMBO J. 20, 5568–5577 (2001).
Iversen, T. G., Skretting, G., Van Deurs, B. & Sandvig, K. Clathrin-coated pits with long, dynamin-wrapped necks upon expression of a clathrin antisense RNA. Proc. Natl Acad. Sci. USA 100, 5175–5180 (2003).
Kessell, I., Holst, B. D. & Roth, T. F. Membranous intermediates in endocytosis are labile, as shown in a temperature-sensitive mutant. Proc. Natl Acad. Sci. USA 86, 4968–4972 (1989).
Koenig, J. H. & Ikeda, K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J. Neurosci. 9, 3844–3860 (1989). Analysis of the shibire phenotype showing temperature-dependent reversible depletion and reformation of synaptic vesicles.
Kawasaki, F., Hazen, M. & Ordway, R. W. Fast synaptic fatigue in shibire mutants reveals a rapid requirement for dynamin in synaptic vesicle membrane trafficking. Nature Neurosci. 3, 859–860 (2000).
Kang, B. H., Busse, J. S. & Bednarek, S. Y. Members of the Arabidopsis dynamin-like gene family, ADL1, are essential for plant cytokinesis and polarized cell growth. Plant Cell 15, 899–913 (2003).
Kang, B. H., Rancour, D. M. & Bednarek, S. Y. The dynamin-like protein ADL1C is essential for plasma membrane maintenance during pollen maturation. Plant J. 35, 1–15 (2003).
Arimura, S. & Tsutsumi, N. A dynamin-like protein (ADL2b), rather than FtsZ, is involved in Arabidopsis mitochondrial division. Proc. Natl Acad. Sci. USA 99, 5727–5731 (2002).
Lam, B. C., Sage, T. L., Bianchi, F. & Blumwald, E. Regulation of ADL6 activity by its associated molecular network. Plant J. 31, 565–576 (2002).
Jin, J. B. et al. A new dynamin-like protein, ADL6, is involved in trafficking from the trans-Golgi network to the central vacuole in Arabidopsis. Plant Cell 13, 1511–1526 (2001).
Nishida, K. et al. Dynamic recruitment of dynamin for final mitochondrial severance in a primitive red alga. Proc. Natl Acad. Sci. USA 100, 2146–2151 (2003).
Lee, E. & De Camilli, P. Dynamin at actin tails. Proc. Natl Acad. Sci. USA 99, 161–166 (2002).
Qualmann, B., Roos, J., DiGregorio, P. J. & Kelly, R. B. Syndapin I, a synaptic dynamin-binding protein that associates with the neural Wiskott–Aldrich syndrome protein. Mol. Biol. Cell 10, 501–513 (1999).
Olichon, A. et al. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J. Biol. Chem. 278, 7743–7746 (2003).
Delettre, C. et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nature Genet. 26, 207–210 (2000).
Alexander, C. et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nature Genet. 26, 211–215 (2000). References 125 and 126 show the link between dominant optic atrophy (DOA) and mutations in a dynamin-superfamily member OPA1.
Toomes, C. et al. Spectrum, frequency and penetrance of OPA1 mutations in dominant optic atrophy. Hum. Mol. Genet. 10, 1369–1378 (2001).
Pesch, U. E. et al. OPA1 mutations in patients with autosomal dominant optic atrophy and evidence for semi-dominant inheritance. Hum. Mol. Genet. 10, 1359–1368 (2001).
Thiselton, D. L. et al. A comprehensive survey of mutations in the OPA1 gene in patients with autosomal dominant optic atrophy. Invest. Ophthalmol. Vis. Sci. 43, 1715–1724 (2002).
Delettre, C., Lenaers, G., Pelloquin, L., Belenguer, P. & Hamel, C. P. OPA1 (Kjer type) dominant optic atrophy: a novel mitochondrial disease. Mol. Genet. Metab. 75, 97–107 (2002).
Marchbank, N. J. et al. Deletion of the OPA1 gene in a dominant optic atrophy family: evidence that haploinsufficiency is the cause of disease. J. Med. Genet. 39, e47 (2002).
Carr, J. F. & Hinshaw, J. E. Dynamin assembles into spirals under physiological salt conditions upon the addition of GDP and γ-phosphate analogues. J. Biol. Chem. 272, 28030–28035 (1997).
Owen, D. J. et al. Crystal structure of the amphiphysin-2 SH3 domain and its role in the prevention of dynamin ring formation. EMBO J. 17, 5273–5285 (1998).
Kochs, G., Janzen, C., Hohenberg, H. & Haller, O. Antivirally active MxA protein sequesters La Crosse virus nucleocapsid protein into perinuclear complexes. Proc. Natl Acad. Sci. USA 99, 3153–3158 (2002).
Mozdy, A. D., McCaffery, J. M. & Shaw, J. M. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J. Cell Biol. 151, 367–380 (2000).
Binns, D. D. et al. The mechanism of GTP hydrolysis by dynamin II: a transient kinetic study. Biochemistry 39, 7188–7196 (2000).
Richter, M. F., Schwemmle, M., Herrmann, C., Wittinghofer, A. & Staeheli, P. Interferon-induced MxA protein. GTP binding and GTP hydrolysis properties. J. Biol. Chem. 270, 13512–13517 (1995).
Schwemmle, M. & Staeheli, P. The interferon-induced 67-kDa guanylate-binding protein (hGBP1) is a GTPase that converts GTP to GMP. J. Biol. Chem. 269, 11299–11305 (1994).
Praefcke, G. J., Geyer, M., Schwemmle, M., Robert Kalbitzer, H. & Herrmann, C. Nucleotide-binding characteristics of human guanylate-binding protein 1 (hGBP1) and identification of the third GTP-binding motif. J. Mol. Biol. 292, 321–332 (1999).
Schweins, T. et al. Substrate-assisted catalysis as a mechanism for GTP hydrolysis of p21ras and other GTP-binding proteins. Nature Struct. Biol. 2, 36–44 (1995).
John, J. et al. Kinetics of interaction of nucleotides with nucleotide-free H-ras p21. Biochemistry 29, 6058–6065 (1990).
Berman, D. M., Wilkie, T. M. & Gilman, A. G. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein α subunits. Cell 86, 445–452 (1996).
McEwen, D. P., Gee, K. R., Kang, H. C. & Neubig, R. R. Fluorescent BODIPY-GTP analogs: real-time measurement of nucleotide binding to G proteins. Anal. Biochem. 291, 109–117 (2001).
Otero, A. D. Transphosphorylation and G protein activation. Biochem. Pharmacol. 39, 1399–1404 (1990).
Bourne, H. R., Sanders, D. A. & McCormick, F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature 348, 125–132 (1990).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Flybase
Interpro
Saccharomyces genome database
Schizosaccharomyces pombe GeneDB
Swiss-Prot
TAIR
FURTHER INFORMATION
Glossary
- SCISSION
-
Cleavage of the vesicle from the parent membrane — as in the use of scissors to sever.
- SNARE
-
(soluble N-ethylmaleimide-sensitive fusion protein attachment protein (SNAP) receptor). SNARE proteins are a family of membrane-tethered coiled-coil proteins that regulate fusion reactions and target specificity in vesicle trafficking. They can be divided into v-SNAREs and t-SNAREs on the basis of their localization.
- CLASSICAL DYNAMINS
-
Dynamins that show sequence homology to the protein described in 1989 as 'dynamin', a microtubule-binding protein. For these classical proteins, the homology extends over the complete length of the protein and they have five distinct domains.
- DYNAMIN-RELATED PROTEINS
-
Dynamins that lack one or more domains or have additional domains that are not present in classical dynamins. Some of these proteins might be functionally indistinguishable from classical dynamins.
- CLATHRIN-COATED VESICLE
-
(CCV). Transport vesicles that bud with the aid of a coat protein known as clathrin.
- CAVEOLAE
-
Flask-shaped invaginations of the plasma membrane that are coated with the protein caveolin. Caveolae are endocytosed in a clathrin-independent manner.
- DYNAMINS
-
Members of the dynamin superfamily, which include the dynamin-related proteins, Mx proteins and GBP/atlastin.
- PLECKSTRIN-HOMOLOGY (PH) DOMAIN
-
A protein module of ∼100 amino acids that is present in a range of proteins. Different PH domains interact with various phospholipids and are therefore involved in the targeting of the proteins.
- SRC-HOMOLOGY-3 (SH3) DOMAIN
-
A protein module of ∼80 amino acids that is present in a range of proteins and was first identified in the protein kinase Src. SH3 domains interact with proline-rich sequences that usually contain a PxxxPxR motif.
- CLATHRIN-COATED PIT
-
(CCP). The initial site of invagination of a clathrin-coated vesicle.
- FISSION
-
The breakage of one object into parts — for example, fission of the lipid membrane.
- PHRAGMOPLASTIN
-
Dynamin-like protein found at the phragmoplast, which is the microtubular network in dividing plant cells that transports Golgi-derived vesicles to the cell plate.
- CELL PLATE
-
A flat, membrane-bound incipient cell wall at the division plane of a plant cell. The cell plate is formed by fusion and tubulation of Golgi-derived vesicles, which results in the outward expansion, and finally fusion, of the vesicles with the side walls.
- ACTIVE ZONE
-
A specialized area of the presynaptic plasma membrane where synaptic vesicles fuse and release their neurotransmitter content.
- SWITCH REGIONS
-
Regions of nucleotide-binding proteins that have different conformations in the triphosphate- compared to the diphosphate-bound state.
- CRISTAE
-
The inward folds of the inner mitochondrial membrane that increase its surface area.
- PLASTIDS
-
Organelles that are found in eukaryotic plant cytoplasm. They all contain DNA and are surrounded by a double membrane.
- RNA INTERFERENCE
-
(RNAi). A form of post-transcriptional gene silencing in which expression or transfection of double-stranded RNA induces degradation — by nucleases — of the homologous endogenous transcripts. This mimics the effect of the reduction, or loss, of gene activity.
- CLEAVAGE FURROW
-
An invagination of the plasma membrane in the division plane of an animal cell that contains a contractile ring, and which leads to scission of the daughter cells.
Rights and permissions
About this article
Cite this article
Praefcke, G., McMahon, H. The dynamin superfamily: universal membrane tubulation and fission molecules?. Nat Rev Mol Cell Biol 5, 133–147 (2004). https://doi.org/10.1038/nrm1313
Issue Date:
DOI: https://doi.org/10.1038/nrm1313
This article is cited by
-
The HEAT repeat protein HPO-27 is a lysosome fission factor
Nature (2024)
-
Dynamin-dependent entry of Chlamydia trachomatis is sequentially regulated by the effectors TarP and TmeA
Nature Communications (2024)
-
Identification and Expression Analysis of Soybean (Glycine max L.) Dynamin Genes Reveal Their Involvements in Plant Development and Stress Response
Tropical Plant Biology (2024)
-
LRRK2 is involved in the chemotaxis of neutrophils and differentiated HL-60 cells, and the inhibition of LRRK2 kinase activity increases fMLP-induced chemotactic activity
Cell Communication and Signaling (2023)
-
Fowl adenovirus serotype 4 enters leghorn male hepatocellular cells via the clathrin-mediated endocytosis pathway
Veterinary Research (2023)