Key Points
-
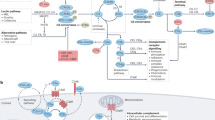
Lectins, which are carbohydrate-binding proteins, are important in innate immunity because they are able to recognize a wide range of pathogens. Two classes of collagenous lectin — mannose-binding lectin (MBL) and ficolins — can activate the complement system, which was identified originally as an antibody-dependent effector system.
-
The complement system, which consists of more than 30 plasma and cell-surface proteins, is activated by three pathways — the classical, lectin and alternative pathways — which lead to the generation of opsonins and pathogen destruction.
-
The classical pathway is activated by binding of the first complement component — which consists of C1q and two serine proteases (C1r and C1s) — to immune complexes, whereas the lectin pathway is activated by the recognition of carbohydrates on pathogens through MBL and ficolins, which are associated with novel serine proteases.
-
MBL-associated serine proteases (MASPs), which share domain structure and several functions with the classical-pathway proteases C1r and C1s, are the proteolytic enzymes that are responsible for activation of the lectin pathway.
-
The ascidian complement system — which consists of MBL-like lectin, ficolins, two MASPs, C3 and C3 receptor — functions in an opsonic manner, and it constitutes a primordial complement system that corresponds to the mammalian lectin pathway.
-
Structural and functional similarities between the lectin–protease complex in the lectin pathway and the first component of the classical complement pathway indicate that the primitive lectin pathway in innate immunity might have evolved into the classical pathway to serve as an effector system of acquired immunity.
Abstract
Discrimination between self and non-self by lectins (carbohydrate-binding proteins) is a strategy of innate immunity that is found in both vertebrates and invertebrates. In vertebrates, immune recognition mediated by ficolins (lectins that consist of a fibrinogen-like and a collagen-like domain), as well as by mannose-binding lectins, triggers the activation of the complement system, which results in the activation of novel serine proteases. The presence of a similar lectin-based complement system in ascidians, our closest invertebrate relatives, indicates that the complement system probably had a pivotal role in innate immunity before the evolution of an adaptive immune system in jawed vertebrates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hoffmann, J. A., Kafatos, F. C., Janeway, C. A. & Ezekowitz, R. A. Phylogenetic perspectives in innate immunity. Science 284, 1313–1318 (1999).
Medzhitov, R. & Janeway, C. Jr. Innate immune recognition: mechanisms and pathways. Immunol. Rev. 173, 89–97 (2000).
Walport, M. J. Complement. First of two parts. N. Engl. J. Med. 344, 1058–1066 (2001).
Walport, M. J. Complement. Second of two parts. N. Engl. J. Med. 344, 1140–1144 (2001).References 3 and 4 provide an excellent review of the complement system. They focus on recent advances in the role of complement in disease, and the interaction between innate and adaptive immunity.
Kishore, U. & Reid, K. B. C1q: structure, function and receptors. Immunopharmacology 49, 159–170 (2000).
Mizuno, Y., Kozutsumi, Y., Kawasaki, T. & Yamashina, I. Isolation and characterization of a mannan-binding protein from rat liver. J. Biol. Chem. 256, 4247–4252 (1981).
Drickamer, K., Dordal, M. S. & Reynolds, L. Mannose-binding proteins isolated from rat liver contain carbohydrate-recognition domains linked to collagenous tails. Complete primary structures and homology with pulmonary surfactant apoprotein. J. Biol. Chem. 261, 6878–6887 (1986).
Ezekowitz, R. A., Day, L. E. & Herman, G. A. A human mannose-binding protein is an acute-phase reactant that shares sequence homology with other vertebrate lectins. J. Exp. Med. 167, 1034–1046 (1988).
Turner, M. W. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol. Today 17, 532–540 (1996).
Super, M., Thiel, S., Lu, J., Levinsky, R. J. & Turner, M. W. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet 2, 1236–1239 (1989).
Summerfield, J. A. et al. Mannose-binding protein gene mutations associated with unusual and severe infections in adults. Lancet 345, 886–889 (1995).
Neth, O. et al. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect. Immun. 68, 688–693 (2000).
Jack, D. L., Klein, N. J. & Turner, M. W. Mannose-binding lectin: targeting the microbial world for complement attack and opsonophagocytosis. Immunol. Rev. 180, 86–99 (2001).
Holmskov, U., Malhotra, R., Sim, R. B. & Jensenius, J. C. Collectins: collagenous C-type lectins of the innate immune defense system. Immunol. Today 15, 67–74 (1994).
Dahl, M. R. et al. MASP-3 and its association with distinct complexes of the mannan-binding lectin complement-activation pathway. Immunity 15, 127–315 (2001).This study shows that human MBL is associated with a third serine protease termed MASP3, which is generated through alternative splicing of the MASP1/3 gene. Another important finding is that different MBL oligomers have distinct MASP composition and biological activities, as shown by the association of MASP1, sMAP and C3 activation with smaller MBL oligomers.
Weis, W. I., Drickamer, K. & Hendrickson, W. A. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature 360, 127–134 (1992).
Drickamer, K. Engineering galactose-binding activity into a C-type mannose-binding protein. Nature 360, 183–186 (1992).
Sheriff, S., Chang, C. Y. & Ezekowitz, R. A. Human mannose-binding protein carbohydrate-recognition domain trimerizes through a triple α-helical coiled-coil. Nature Struct. Biol. 1, 789–794 (1994).
Laursen, S. B. & Nielsen, O. L. Mannan-binding lectin (MBL) in chickens: molecular and functional aspects. Dev. Comp. Immunol. 24, 85–101 (2000).
Vitved, L. et al. The homologue of mannose-binding lectin in the carp family Cyprinidae is expressed at high level in spleen, and the deduced primary structure predicts affinity for galactose. Immunogenetics 51, 955–964 (2000).
Matsushita, M. & Fujita, T. Ficolins and the lectin complement pathway. Immunol. Rev. 180, 78–85 (2001).
Ichijo, H. et al. Molecular cloning and characterization of ficolin, a multimeric protein with fibrinogen- and collagen-like domains. J. Biol. Chem. 268, 14505–14513 (1993).
Edgar, P. F. Hucolin, a new corticosteroid-binding protein from human plasma with structural similarities to ficolins, transforming growth factor-β-1-binding proteins. FEBS Lett. 375, 159–161 (1995).
Matsushita, M. et al. A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J. Biol. Chem. 271, 2448–2454 (1996).
Lu, J., Tay, P. N., Kon, O. L. & Reid, K. B. Human ficolin: cDNA cloning, demonstration of peripheral-blood leucocytes as the major site of synthesis and assignment of the gene to chromosome 9. Biochem. J. 313, 473–478 (1996).
Endo, Y., Sato, Y., Matsushita, M. & Fujita, T. Cloning and characterization of the human lectin P35 gene and its related gene. Genomics 36, 515–521 (1996).
Harumiya, S. et al. Characterization of ficolins as novel elastin-binding proteins and molecular cloning of human ficolin-1. J. Biochem. (Tokyo) 120, 745–751 (1996).
Sugimoto, R. et al. Cloning and characterization of the Hakata antigen, a member of the ficolin/opsonin p35 lectin family. J. Biol. Chem. 273, 20721–20727 (1998).
Fujimori, Y. et al. Molecular cloning and characterization of mouse ficolin-A. Biochem. Biophys. Res. Commun. 244, 796–800 (1998).
Ohashi, T. & Erickson, H. P. Two oligomeric forms of plasma ficolin have differential lectin activity. J. Biol. Chem. 272, 14220–14226 (1997).
Omori-Satoh, T., Yamakawa, Y. & Mebs, D. The antihemorrhagic factor, erinacin, from the European hedgehog (Erinaceus europaeus), a metalloprotease inhibitor of large molecular size possessing ficolin/opsonin P35 lectin domains. Toxicon 38, 1561–1580 (2000).
Kenjo, A. et al. Cloning and characterization of novel ficolins from the solitary ascidian, Halocynthia roretzi. J. Biol. Chem. 276, 19959–19965 (2001).The first description of ficolins in invertebrates as GlcNAc-binding lectins, which are homologues of mammalian ficolins. The presence of ficolins in invertebrates indicates their crucial role in innate immunity.
Akaiwa, M. et al. Hakata antigen, a new member of the ficolin/opsonin p35 family, is a novel human lectin secreted into bronchus/alveolus and bile. J. Histochem. Cytochem. 47, 777–786 (1999).
Teh, C., Le, Y., Lee, S. H. & Lu, J. M-ficolin is expressed on monocytes and is a lectin binding to N-acetyl-d-glucosamine and mediates monocyte adhesion and phagocytosis of Escherichia coli. Immunology 101, 225–232 (2000).
Matsushita, M., Endo, Y. & Fujita, T. Cutting edge: complement-activating complex of ficolin and mannose-binding-lectin-associated serine protease. J. Immunol. 164, 2281–2284 (2000).This paper describes L-ficolin/P35 as another lectin that is able to mediate complement activation through association with MASPs.
Gokudan, S. et al. Horseshoe crab acetyl-group-recognizing lectins involved in innate immunity are structurally related to fibrinogen. Proc. Natl Acad. Sci. USA 96, 10086–10091 (1999).
Kairies, N. et al. The 2.0 Å crystal structure of tachylectin 5A provides evidence for the common origin of the innate immunity and the blood coagulation systems. Proc. Natl Acad. Sci. USA 98, 13519–13524 (2001).
Matsushita, M. & Fujita, T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J. Exp. Med. 176, 1497–1502 (1992).
Sato, T., Endo, Y., Matsushita, M. & Fujita, T. Molecular characterization of a novel serine protease involved in activation of the complement system by mannose-binding protein. Int. Immunol. 6, 665–669 (1994).
Takada, F., Takayama, Y., Hatsuse, H. & Kawakami, M. A new member of the C1s family of complement proteins found in a bactericidal factor, Ra-reactive factor, in human serum. Biochem. Biophys. Res. Commun. 196, 1003–1009 (1993).
Thiel, S. et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature 386, 506–510 (1997).
Ji, Y. H. et al. Activation of the C4 and C2 components of complement by a proteinase in serum bactericidal factor, Ra-reactive factor. J. Immunol. 150, 571–578 (1993).
Matsushita, M., Takahashi, A., Hatsuse, H., Kawakami, M. & Fujita, T. Human mannose-binding protein is identical to a component of Ra-reactive factor. Biochem. Biophys. Res. Commun. 183, 645–651 (1992).
Takahashi, M., Endo, Y., Fujita, T. & Matsushita, M. A truncated form of mannose-binding lectin-associated serine protease (MASP)-2 expressed by alternative polyadenylation is a component of the lectin complement pathway. Int. Immunol. 11, 859–863 (1999).
Stover, C. M., Schwaeble, W. J., Lynch, N. J., Thiel, S. & Speicher, M. R. Assignment of the gene encoding mannan-binding-lectin-associated serine protease 2 (MASP2) to human chromosome 1p36.3→p36.2 by in situ hybridization and somatic-cell hybrid analysis. Cytogenet. Cell Genet. 84, 148–149 (1999).
Thiel, S. et al. Interaction of C1q and mannan-binding lectin (MBL) with C1r, C1s, MBL-associated serine proteases 1 and 2, and the MBL-associated protein MAp19. J. Immunol. 165, 878–887 (2000).
Gadjeva, M., Thiel, S. & Jensenius, J. C. The mannan-binding-lectin pathway of the innate immune response. Curr. Opin. Immunol. 13, 74–78 (2001).
Matsushita, M., Thiel, S., Jensenius, J. C., Terai, I. & Fujita, T. Proteolytic activities of two types of mannose-binding-lectin-associated serine protease. J. Immunol. 165, 2637–2642 (2000).
Vorup-Jensen, T. et al. Distinct pathways of mannan-binding lectin (MBL)- and C1-complex autoactivation revealed by reconstitution of MBL with recombinant MBL-associated serine protease 2. J. Immunol. 165, 2093–2100 (2000).
Wallis, R. & Dodd, R. B. Interaction of mannose-binding protein with associated serine proteases: effects of naturally occurring mutations. J. Biol. Chem. 275, 30962–30969 (2000).
Chen, C. B. & Wallis, R. Stoichiometry of complexes between mannose-binding protein and its associated serine proteases. Defining functional units for complement activation. J. Biol. Chem. 276, 25894–25902 (2001).
Thielens, N. M. et al. Interaction properties of human mannan-binding lectin (MBL)-associated serine proteases 1 and 2, MBL-associated protein 19 and MBL. J. Immunol. 166, 5068–5077 (2001).References 49–52 describe the molecular interactions between MASP1, MASP2 and sMAP/MAp19 in association with MBL.
Matsushita, M. & Fujita, T. Cleavage of the third component of complement (C3) by mannose-binding-protein-associated serine protease (MASP) with subsequent complement activation. Immunobiology 194, 443–448 (1995).
Rossi, V. et al. Substrate specificities of recombinant mannan-binding-lectin-associated serine proteases 1 and 2. J. Biol. Chem. 276, 40880–40887 (2001).
Endo, Y., Sato, T., Matsushita, M. & Fujita, T. Exon structure of the gene encoding the human mannose-binding-protein-associated serine protease light chain: comparison with complement C1r and C1s genes. Int. Immunol. 8, 1355–1358 (1996).
Endo, Y. et al. Two lineages of mannose-binding-lectin-associated serine protease (MASP) in vertebrates. J. Immunol. 161, 4924–4930 (1998).
Matsushita, M., Endo, Y., Nonaka, M. & Fujita, T. Complement-related serine proteases in tunicates and vertebrates. Curr. Opin. Immunol. 10, 29–35 (1998).
Smith, L. C., Chang, L., Britten, R. J. & Davidson, E. H. Sea urchin genes expressed in activated coelomocytes are identified by expressed sequence tags. Complement homologues and other putative immune response genes suggest immune system homology within the deuterostomes. J. Immunol. 156, 593–602 (1996).
Smith, L. C., Shih, C. S. & Dachenhausen, S. G. Coelomocytes express SpBf, a homologue of factor B, the second component in the sea urchin complement system. J. Immunol. 161, 6784–6793 (1998).
Smith, L. C., Clow, L. A. & Terwilliger, D. P. The ancestral complement system in sea urchins. Immunol. Rev. 180, 16–34 (2001).
Nonaka, M. Evolution of the complement system. Curr. Opin. Immunol. 13, 69–73 (2001).An excellent, recent review of the molecular evolution of the complement system.
Sekine, H. et al. An ancient lectin-dependent complement system in an ascidian: novel lectin isolated from the plasma of the solitary ascidian, Halocynthia roretzi. J. Immunol. 167, 4504–4510 (2001).This study describes the presence of glucose-binding lectin (GBL) in the plasma of the solitary ascidian, which has a carbohydrate-recognition domain but lacks a collagen-like domain. The important finding is that the GBL–MASPs complex activates C3, leading to C3-dependent phagocytosis, which indicates that the primitive complement system consists of the lectin–proteases complex and C3.
Ji, X., Azumi, K., Sasaki, M. & Nonaka, M. Ancient origin of the complement lectin pathway revealed by molecular cloning of mannan-binding-protein-associated serine protease from a urochordate, the Japanese ascidian, Halocynthia roretzi. Proc. Natl Acad. Sci. USA 94, 6340–6345 (1997).
Nonaka, M. et al. Opsonic complement component C3 in the solitary ascidian, Halocynthia roretzi. J. Immunol. 162, 387–391 (1999).
Miyazawa, S., Azumi, K. & Nonaka, M. Cloning and characterization of integrin α-subunits from the solitary ascidian, Halocynthia roretzi. J. Immunol. 166, 1710–1715 (2001).This study describes the presence of C3 receptor in the ascidian — which is homologous to human C3 receptor, CR3 or CR4 — and that this receptor is involved in the phagocytosis of yeast by ascidian haemocytes.
Nair, S. V. et al. A collectin-like protein from tunicates. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 125, 279–289 (2000).
Vasta, G. R., Quesenberry, M., Ahmed, H. & O'Leary, N. C-type lectins and galectins mediate innate and adaptive immune functions: their roles in the complement activation pathway. Dev. Comp. Immunol. 23, 401–420 (1999).
Bulgakov, A. A. et al. Isolation and properties of a mannan-binding lectin from the coelomic fluid of the holothurian Cucumaria japonica. Biochemistry (Moscow) 65, 933–939 (2000).
Horstmann, R. D., Pangburn, M. K. & Muller-Eberhard, H. J. Species specificity of recognition by the alternative pathway of complement. J. Immunol. 134, 1101–1104 (1985).
Kuhlman, M., Joiner, K. & Ezekowitz, R. A. The human mannose-binding protein functions as an opsonin. J. Exp. Med. 169, 1733–1745 (1989).
Acknowledgements
This work was supported by grants from the Ministry of Education, Culture, Sport, Science and Technology, and the Japanese Society for the Promotion of Science. I thank Y. Endo, M. Matsushita and M. Takahashi for helpful discussions and sharing of unpublished data.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
InterPro
LocusLink
Glossary
- C-TYPE LECTIN
-
A calcium-dependent animal lectin that is a carbohydrate-binding protein. The binding activity of a C-type lectin is based on the structure of the carbohydrate-recognition domain, which is highly conserved among this family. Calcium is essential not only for the carbohydrate binding itself, but also for the structural maintenance of this domain.
- COLLECTIN
-
A C-type lectin that has a collagen-like domain. One group, the secreted lectins, consists of mannose-binding lectin, bovine conglutinin and collectin 43 in blood, and the two mucosal-associated proteins surfactant proteins A and D. The other group consists of the newly discovered, non-secreted-type collectin liver-1 and membrane-type collectin placenta-1.
Rights and permissions
About this article
Cite this article
Fujita, T. Evolution of the lectin–complement pathway and its role in innate immunity. Nat Rev Immunol 2, 346–353 (2002). https://doi.org/10.1038/nri800
Issue Date:
DOI: https://doi.org/10.1038/nri800