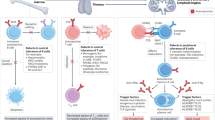
Key Points
-
Cytokines have essential roles in immune cell development, immunoregulation and immune effector functions.
-
Cytokines such as interleukin (IL)-2, tumour-necrosis factor (TNF) and the interferons are well known to have immunostimulatory and pro-inflammatory actions. However, the same cytokines also have unexpected, but essential, immunosuppressive actions.
-
IL-2 has essential functions in constraining lymphocyte growth by promoting apoptosis. Regulatory T cells that express the IL-2 receptor γ-chain have also been intensively studied. Deficiency of these cells can result in autoimmunity, but the exact role of IL-2 in the physiology of these cells is unknown.
-
Despite TNF's role as the prototypic cytokine that mediates proinflammatory responses, it is now clear that its in vivo role is complex. Experimental models of disease show that immune-mediated disease, including arthritis, can occur in the absence of TNF. Models of diabetes have shown that TNF can worsen or improve disease, depending on the timing and duration of exposure to this cytokine.
-
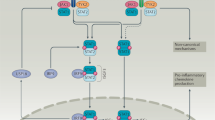
Interferons have essential functions in host defence and promote cell-mediated immunity. Type 1 interferons, in particular, have been used to treat autoimmune diseases, including those characterized by T-helper (TH)1-mediated pathology. Type 1 interferons can inhibit secretion of IL-12 and inhibit its action. Type 2 interferon (interferon-γ) and other cytokines upregulate the expression of a class of feedback inhibitors known as suppressors of cytokine signalling (SOCS). Mice deficient in Socs1 have fatal, interferon-dependent, inflammatory disease.
Abstract
Cytokines have crucial functions in the development, differentiation and regulation of immune cells. As a result, dysregulation of cytokine production or action is thought to have a central role in the development of autoimmunity and autoimmune disease. Some cytokines, such as interleukin-2, tumour-necrosis factor and interferons — ostensibly, the 'bad guys' in terms of disease pathogenesis — are well known for the promotion of immune and inflammatory responses. However, these cytokines also have crucial immunosuppressive functions and so, paradoxically, can also be 'good guys'. The balance between the pro-inflammatory and immunosuppressive functions of these well-known cytokines and the implications for the pathogenesis of autoimmune disease is the focus of this review.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Davidson, A. & Diamond, B. Autoimmune diseases. N. Engl. J. Med. 345, 340–350 (2001).
Marrack, P., Kappler, J. & Kotzin, B. L. Autoimmune disease: why and where it occurs. Nature Med. 7, 899–905 (2001).
Falcone, M. & Sarvetnick, N. Cytokines that regulate autoimmune responses. Curr. Opin. Immunol. 11, 670–676 (1999).
Ioannou, Y. & Isenberg, D. A. Current evidence for the induction of autoimmune rheumatic manifestations by cytokine therapy. Arthritis Rheum. 43, 1431–1442 (2000).
Glimcher, L. H. & Murphy, K. M. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 14, 1693–1711 (2000).
Gadina, M. et al. Signaling by type I and II cytokine receptors: ten years after. Curr. Opin. Immunol. 13, 363–373 (2001).
Rozzo, S. J. et al. Evidence for an interferon-inducible gene, Ifi202, in the susceptibility to systemic lupus. Immunity 15, 435–443 (2001). A very recent and exciting development in the field of autoimmunity. Extensive efforts to map genes associated with autoimmune disease are beginning to pay off, but raise new mechanistic questions.
Morahan, G. et al. Linkage disequilibrium of a type 1 diabetes susceptibility locus with a regulatory IL12B allele. Nature Genet. 27, 218–221 (2001).
Akira, S. Roles of STAT3 defined by tissue-specific gene targeting. Oncogene 19, 2607–2611 (2000).
Stene, L. C. & Nafstad, P. Relation between occurrence of type 1 diabetes and asthma. Lancet 357, 607–608 (2001).
Horak, I., Lohler, J., Ma, A. & Smith, K. A. Interleukin-2 deficient mice: a new model to study autoimmunity and self-tolerance. Immunol. Rev. 148, 35–44 (1995).
Refaeli, Y., Van, P. L. & Abbas, A. K. Genetic models of abnormal apoptosis in lymphocytes. Immunol. Rev. 169, 273–282 (1999). An excellent review that includes detailed discussion of how IL-2 can promote apoptosis.
Lenardo, M. J. Interleukin-2 programs mouse αβ T lymphocytes for apoptosis. Nature 353, 858–861 (1991). A landmark paper demonstrating the counterintuitive effects of IL-2.
Bleesing, J. J., Straus, S. E. & Fleisher, T. A. Autoimmune lymphoproliferative syndrome. A human disorder of abnormal lymphocyte survival. Pediatr. Clin. North. Am. 47, 1291–1310 (2000).
Refaeli, Y., Van Parijs, L., London, C. A., Tschopp, J. & Abbas, A. K. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity 8, 615–623 (1998).
Van Parijs, L. et al. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity 11, 281–288 (1999).
Sakaguchi, S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell 101, 455–458 (2000). A review of CD25+ regulatory by a founder of the field.
Shevach, E. M. Suppressor T cells: rebirth, function and homeostasis. Curr. Biol. 10, R572–R575 (2000).
Wolf, M., Schimpl, A. & Hunig, T. Control of T cell hyperactivation in IL-2-deficient mice by CD4+CD25− and CD4+CD25+ T cells: evidence for two distinct regulatory mechanisms. Eur. J. Immunol. 6, 1637–1645 (2001). | PubMed |
Suzuki, H., Zhou, Y. W., Kato, M., Mak, T. W. & Nakashima, I. Normal regulatory α/β T cells effectively eliminate abnormally activated T cells lacking the interleukin 2 receptor β in vivo. J. Exp. Med. 190, 1561–1572 (1999).
Oosterwegel, M. A., Greenwald, R. J., Mandelbrot, D. A., Lorsbach, R. B. & Sharpe, A. H. CTLA-4 and T cell activation. Curr. Opin. Immunol. 11, 294–300 (1999).
Skapenko, A., Lipsky, P. E., Kraetsch, H. G., Kalden, J. R. & Schulze-Koops, H. Antigen-independent TH2 cell differentiation by stimulation of CD28: regulation via IL-4 gene expression and mitogen-activated protein kinase activation. J. Immunol. 166, 4283–4292 (2001).
Hugot, J. P. et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411, 599–603 (2001).
Ogura, Y. et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411, 603–606 (2001).
Miceli-Richard, C. et al. CARD15 mutations in Blau syndrome. Nature Genet. 29, 19–20 (2001). References 23–25 are breakthroughs in the identification of a gene associated with a relatively common human autoimmune disease, Crohn's disease. Curiously, mutations of the same gene can also be associated with a very different autoimmune disease with distinct pathological features. But what, if any, is the connection with the colitis observed in IL-2 deficient mice?
Kollias, G., Douni, E., Kassiotis, G. & Kontoyiannis, D. The function of tumour necrosis factor and receptors in models of multi-organ inflammation, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. Ann Rheum Dis 58 (Suppl 1), 132–139 (1999). | PubMed |
Owens, T., Wekerle, H. & Antel, J. Genetic models for CNS inflammation. Nature Med. 7, 161–166 (2001).
Campbell, I. K., O'Donnell, K., Lawlor, K. E. & Wicks, I. P. Severe inflammatory arthritis and lymphadenopathy in the absence of TNF. J. Clin. Invest. 107, 1519–1527 (2001). A provocative recent study using a model of arthritis in which TNF-dependent pathology is a prominent feature, which shows that inflammation and, interestingly, adenopathy occurred in the absence of TNF.
Green, E. A. & Flavell, R. A. The initiation of autoimmune diabetes. Curr. Opin. Immunol. 11, 663–669 (1999).
Green, E. A. & Flavell, R. A. The temporal importance of TNFα expression in the development of diabetes. Immunity 12, 459–469 (2000).
Christen, U. et al. A dual role for TNF-α in type 1 diabetes: islet-specific expression abrogates the ongoing autoimmune process when induced late but not early during pathogenesis. J. Immunol. 166, 7023–7032 (2001). References 30 and 31 are vivid examples that the timing and duration of exposure to TNF might make a big difference.
Cope, A. P. Regulation of autoimmunity by proinflammatory cytokines. Curr. Opin. Immunol. 10, 669–676 (1998).
Lauer, G. M. & Walker, B. D. Hepatitis C virus infection. N. Engl. J. Med. 345, 41–52 (2001).
Holland, S. M. Immunotherapy of mycobacterial infections. Semin. Respir. Infect. 16, 47–59 (2001).
Karp, C. L., van Boxel-Dezaire, A. H., Byrnes, A. A. & Nagelkerken, L. Interferon-β in multiple sclerosis: altering the balance of interleukin-12 and interleukin-10? Curr. Opin. Neurol. 14, 361–368 (2001).
O'Shea, J. J. & Visconti, R. Type 1 IFNs and regulation of TH1 responses: enigmas both resolved and emerge. Nature Immunol. 1, 17–19 (2000).
Biron, C. A. Interferons α and β as immune regulators — a new look. Immunity 14, 661–664 (2001).
Levings, M. K. IFN-α and IL-10 induce the differentiation of human type 1 T regulatory cells. J. Immunol. 166, 5530–5539 (2001).
Lighvani, A. A. et al. T-bet is rapidly induced by interferon-γ in lymphoid and myeloid cells. Proc. Natl Acad. Sci. USA (in the press).
Matthys, P., Vermeire, K. & Billiau, A. Mac-1+ myelopoiesis induced by CFA: a clue to the paradoxical effects of IFN-γ in autoimmune disease models. Trends Immunol. 22, 367–371 (2001).
Roifman, C. M. Human IL-2 receptor α chain deficiency. Pediatr. Res. 48, 6–11 (2000).
Grunebaum, E., Zhang, J., Dadi, H. & Roifman, C. M. Haemophagocytic lymphohistiocytosis in X-linked severe combined immunodeficiency. Br. J. Haematol. 108, 834–837 (2000).
Frucht, D. M. et al. Unexpected and variable phenotypes in a family with JAK3 deficiency. Genes Immun. (in the press).
Moriggl, R. et al. Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity 10, 249–259 (1999).
Crispin, J. C. & Alcocer-Varela, J. Interleukin-2 and systemic lupus erythematosus — fifteen years later. Lupus 7, 214–222 (1998).
Podolin, P. L. et al. Differential glycosylation of interleukin 2, the molecular basis for the NOD Idd3 type 1 diabetes gene? Cytokine 12, 477–482 (2000).
Todd, J. A. & Wicker, L. S. Genetic protection from the inflammatory disease type 1 diabetes in humans and animal models. Immunity 15, 387–395 (2001).
Acknowledgements
We thank B. Diamond, M. Lenardo, P. Plotz and W. Strober for reading this manuscript and providing helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
autoimmune lymphoproliferative disease
FURTHER INFORMATION
autoimmune disease: pathogenesis
Glossary
- CROHN'S DISEASE
-
One of the two predominant forms of inflammatory bowel disease that afflicts human patients. The pathophysiology is unknown, but is presumed to be autoimmune in nature.
- TOLERANCE
-
Denotes lymphocyte non-responsiveness to antigen, but implies an active process, not simply a passive lack of response.
- PERIPHERAL TOLERANCE
-
This form of tolerance refers to the lack of responsiveness of mature lymphocytes.
- LYMPHADENOPATHY
-
Enlargement of lymph nodes.
- BLAU SYNDROME
-
A rare, autosomal-dominant disorder characterized by granulomatous arthritis, uveitis, skin rash and cranial neuropathy.
- CENTRAL TOLERANCE
-
This form of tolerance refers to the lack of self-responsiveness found as lymphoid cells develop, and is associated with the deletion of autoreactive clones. For T cells, this occurs in the thymus.
Rights and permissions
About this article
Cite this article
O'Shea, J., Ma, A. & Lipsky, P. Cytokines and autoimmunity. Nat Rev Immunol 2, 37–45 (2002). https://doi.org/10.1038/nri702
Issue Date:
DOI: https://doi.org/10.1038/nri702