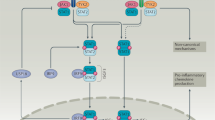
Key Points
-
Most, if not all, cells in humans and mice express the receptor for type I interferons (IFNs). Therefore, these cytokines have a range of direct and indirect effects on various cell types during infection with viruses, bacteria, parasites and fungi.
-
Type I IFNs are important for host defence against viruses, through the induction of antiviral effector molecules that are encoded by IFN-stimulated genes. These IFNs can, however, cause immunopathology in acute viral infections. Conversely, they can lead to immunosuppression and loss of virus control during chronic viral infections.
-
During bacterial infections, low levels of type I IFNs may be required early, to initiate cell-mediated immune responses. By contrast, type I IFNs have been shown to have adverse effects in infections with intracellular bacteria such as Listeria monocytogenes and Mycobacterium tuberculosis.
-
In bacterial infections, high concentrations of type I IFNs may block B cell responses or may lead to the production of immunosuppressive molecules such as interleukin-10.
-
Type I IFNs also antagonize the action of type II IFN (that is, IFNγ) by reducing the responsiveness of macrophages to activation by type II IFN.
-
Another important antagonism is between type I IFNs and interleukin-1. This antagonism was recently shown to be important in M. tuberculosis infection and to be mediated by eicosanoids, in particular prostaglandin E2.
-
Thus, type I IFNs are part of a complex cross-regulatory network, which leads mostly, but not always, to protection of the host against infectious diseases with minimum damage to the host.
Abstract
Type I interferons (IFNs) have diverse effects on innate and adaptive immune cells during infection with viruses, bacteria, parasites and fungi, directly and/or indirectly through the induction of other mediators. Type I IFNs are important for host defence against viruses. However, recently, they have been shown to cause immunopathology in some acute viral infections, such as influenza virus infection. Conversely, they can lead to immunosuppression during chronic viral infections, such as lymphocytic choriomeningitis virus infection. During bacterial infections, low levels of type I IFNs may be required at an early stage, to initiate cell-mediated immune responses. High concentrations of type I IFNs may block B cell responses or lead to the production of immunosuppressive molecules, and such concentrations also reduce the responsiveness of macrophages to activation by IFNγ, as has been shown for infections with Listeria monocytogenes and Mycobacterium tuberculosis. Recent studies in experimental models of tuberculosis have demonstrated that prostaglandin E2 and interleukin-1 inhibit type I IFN expression and its downstream effects, demonstrating that a cross-regulatory network of cytokines operates during infectious diseases to provide protection with minimum damage to the host.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pestka, S., Krause, C. D. & Walter, M. R. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 202, 8–32 (2004).
Schoenborn, J. R. & Wilson, C. B. Regulation of interferon-γ during innate and adaptive immune responses. Adv. Immunol. 96, 41–101 (2007).
O'Brien, T. R., Prokunina-Olsson, L. & Donnelly, R. P. IFN-λ4: the paradoxical new member of the interferon λ family. J. Interferon Cytokine Res. 34, 829–838 (2014).
Prokunina-Olsson, L. et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nature Genet. 45, 164–171 (2013).
Witte, K., Witte, E., Sabat, R. & Wolk, K. IL-28A, IL-28B, and IL-29: promising cytokines with type I interferon-like properties. Cytokine Growth Factor Rev. 21, 237–251 (2010).
Durbin, R. K., Kotenko, S. V. & Durbin, J. E. Interferon induction and function at the mucosal surface. Immunol. Rev. 255, 25–39 (2013).
Yan, N. & Chen, Z. J. Intrinsic antiviral immunity. Nature Immunol. 13, 214–222 (2012).
Goubau, D., Deddouche, S. & Reis e Sousa, C. Cytosolic sensing of viruses. Immunity 38, 855–869 (2013).
Paludan, S. R. & Bowie, A. G. Immune sensing of DNA. Immunity 38, 870–880 (2013).
Leber, J. H. et al. Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen. PLoS Pathog. 4, e6 (2008).
Pandey, A. K. et al. NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog. 5, e1000500 (2009).
Watanabe, T. et al. NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway. J. Clin. Invest. 120, 1645–1662 (2010).
Moreira, L. O. & Zamboni, D. S. NOD1 and NOD2 signaling in infection and inflammation. Front. Immunol. 3, 328 (2012).
Moynagh, P. N. TLR signalling and activation of IRFs: revisiting old friends from the NF-κB pathway. Trends Immunol. 26, 469–476 (2005).
Honda, K., Takaoka, A. & Taniguchi, T. Type I interferon gene induction by the interferon regulatory factor family of transcription factors. Immunity 25, 349–360 (2006).
Tamura, T., Yanai, H., Savitsky, D. & Taniguchi, T. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 26, 535–584 (2008).
Ivashkiv, L. B. & Donlin, L. T. Regulation of type I interferon responses. Nature Rev. Immunol. 14, 36–49 (2014). This review is a perfect prelude to the present review and describes the molecular mechanisms of regulation of type I IFNs in more detail.
Rauch, I., Muller, M. & Decker, T. The regulation of inflammation by interferons and their STATs. JAKSTAT 2, e23820 (2013).
Versteeg, G. A. & Garcia-Sastre, A. Viral tricks to grid-lock the type I interferon system. Curr. Opin. Microbiol. 13, 508–516 (2010).
McNab, F. W., Rajsbaum, R., Stoye, J. P. & O'Garra, A. Tripartite-motif proteins and innate immune regulation. Curr. Opin. Immunol. 23, 46–56 (2011).
Diamond, M. S. & Schoggins, J. W. Host restriction factor screening: let the virus do the work. Cell Host Microbe 14, 229–231 (2013).
Muller, U. et al. Functional role of type I and type II interferons in antiviral defense. Science 264, 1918–1921 (1994).
Haller, O., Arnheiter, H., Gresser, I. & Lindenmann, J. Virus-specific interferon action. Protection of newborn Mx carriers against lethal infection with influenza virus. J. Exp. Med. 154, 199–203 (1981).
Durbin, J. E. et al. Type I IFN modulates innate and specific antiviral immunity. J. Immunol. 164, 4220–4228 (2000).
Garcia-Sastre, A. et al. The role of interferon in influenza virus tissue tropism. J. Virol. 72, 8550–8558 (1998).
Koerner, I., Kochs, G., Kalinke, U., Weiss, S. & Staeheli, P. Protective role of β interferon in host defense against influenza A virus. J. Virol. 81, 2025–2030 (2007).
Price, G. E., Gaszewska-Mastarlarz, A. & Moskophidis, D. The role of α/β and γ interferons in development of immunity to influenza A virus in mice. J. Virol. 74, 3996–4003 (2000).
Mordstein, M. et al. λ Interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J. Virol. 84, 5670–5677 (2010).
Mordstein, M. et al. Interferon-λ contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog. 4, e1000151 (2008). This study demonstrates the redundant roles of type I and type III IFNs in the anti-influenza virus response, clarifying the confusion arising from earlier literature that reported that type I IFNs cannot account for the requirement for STAT1 signalling in protection against influenza virus infection.
Crotta, S. et al. Type I and type III interferons drive redundant amplification loops to induce a transcriptional signature in influenza-infected airway epithelia. PLoS Pathog. 9, e1003773 (2013). This study demonstrates the redundant roles of type I and type III IFN signalling in epithelial cells in the anti-influenza virus response, clarifying the confusion arising from earlier literature over protection against influenza virus infection.
Casanova, J. L., Holland, S. M. & Notarangelo, L. D. Inborn errors of human JAKs and STATs. Immunity 36, 515–528 (2012).
Zhang, S. Y. et al. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-α/β, IFN-γ, and IFN-λ in host defense. Immunol. Rev. 226, 29–40 (2008).
Suppiah, V. et al. IL28B is associated with response to chronic hepatitis C interferon-α and ribavirin therapy. Nature Genet. 41, 1100–1104 (2009).
Tanaka, Y. et al. Genome-wide association of IL28B with response to pegylated interferon-α and ribavirin therapy for chronic hepatitis C. Nature Genet. 41, 1105–1109 (2009).
Ge, D. et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461, 399–401 (2009).
Thomas, D. L. et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 461, 798–801 (2009).
Sandler, N. G. et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature 511, 601–605 (2014).
Everitt, A. R. et al. IFITM3 restricts the morbidity and mortality associated with influenza. Nature 484, 519–523 (2012). This study provided the first evidence of host genetics ( IFITM3 ) contributing to susceptibility to influenza virus infection in humans.
Zhang, Y. H. et al. Interferon-induced transmembrane protein-3 genetic variant rs12252-C is associated with severe influenza in Chinese individuals. Nature Commun. 4, 1418 (2013). This is a follow-up study to reference 38, showing that IFITM3 variants that contribute to the severity of influenza virus infection are predominant in the Chinese population.
Staeheli, P., Grob, R., Meier, E., Sutcliffe, J. G. & Haller, O. Influenza virus-susceptible mice carry Mx genes with a large deletion or a nonsense mutation. Mol. Cell. Biol. 8, 4518–4523 (1988).
Horisberger, M. A., Staeheli, P. & Haller, O. Interferon induces a unique protein in mouse cells bearing a gene for resistance to influenza virus. Proc. Natl Acad. Sci. USA 80, 1910–1914 (1983).
Horby, P., Nguyen, N. Y., Dunstan, S. J. & Baillie, J. K. The role of host genetics in susceptibility to influenza: a systematic review. PLoS ONE 7, e33180 (2012).
Dauer, M. et al. Interferon-α disables dendritic cell precursors: dendritic cells derived from interferon-α-treated monocytes are defective in maturation and T-cell stimulation. Immunology 110, 38–47 (2003).
Lapenta, C. et al. Potent immune response against HIV-1 and protection from virus challenge in hu-PBL-SCID mice immunized with inactivated virus-pulsed dendritic cells generated in the presence of IFN-α. J. Exp. Med. 198, 361–367 (2003).
Santini, S. M. et al. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J. Exp. Med. 191, 1777–1788 (2000).
Santodonato, L. et al. Monocyte-derived dendritic cells generated after a short-term culture with IFN-α and granulocyte-macrophage colony-stimulating factor stimulate a potent Epstein-Barr virus-specific CD8+ T cell response. J. Immunol. 170, 5195–5202 (2003).
Hahm, B., Trifilo, M. J., Zuniga, E. I. & Oldstone, M. B. Viruses evade the immune system through type I interferon-mediated STAT2-dependent, but STAT1-independent, signaling. Immunity 22, 247–257 (2005).
Ito, T. et al. Differential regulation of human blood dendritic cell subsets by IFNs. J. Immunol. 166, 2961–2969 (2001).
Montoya, M. et al. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood 99, 3263–3271 (2002).
Le Bon, A. et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nature Immunol. 4, 1009–1015 (2003).
Le Bon, A. et al. Direct stimulation of T cells by type I IFN enhances the CD8+ T cell response during cross-priming. J. Immunol. 176, 4682–4689 (2006).
Spadaro, F. et al. IFN-α enhances cross-presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood 119, 1407–1417 (2012).
Parlato, S. et al. Expression of CCR-7, MIP-3β, and Th-1 chemokines in type I IFN-induced monocyte-derived dendritic cells: importance for the rapid acquisition of potent migratory and functional activities. Blood 98, 3022–3029 (2001).
Rouzaut, A. et al. Dendritic cells adhere to and transmigrate across lymphatic endothelium in response to IFN-α. Eur. J. Immunol. 40, 3054–3063 (2010).
Gautier, G. et al. A type I interferon autocrine–paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J. Exp. Med. 201, 1435–1446 (2005).
Cousens, L. P., Orange, J. S., Su, H. C. & Biron, C. A. Interferon-α/β inhibition of interleukin 12 and interferon-γ production in vitro and endogenously during viral infection. Proc. Natl Acad. Sci. USA 94, 634–639 (1997).
Dalod, M. et al. Interferon α/β and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J. Exp. Med. 195, 517–528 (2002).
Orange, J. S., Wolf, S. F. & Biron, C. A. Effects of IL-12 on the response and susceptibility to experimental viral infections. J. Immunol. 152, 1253–1264 (1994).
Orange, J. S. et al. Mechanism of interleukin 12-mediated toxicities during experimental viral infections: role of tumor necrosis factor and glucocorticoids. J. Exp. Med. 181, 901–914 (1995).
Le Bon, A. et al. Enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J. Immunol. 176, 2074–2078 (2006).
Havenar-Daughton, C., Kolumam, G. A. & Murali-Krishna, K. The direct action of type I IFN on CD4 T cells is critical for sustaining clonal expansion in response to a viral but not a bacterial infection. J. Immunol. 176, 3315–3319 (2006).
Brinkmann, V., Geiger, T., Alkan, S. & Heusser, C. H. Interferon α increases the frequency of interferon γ-producing human CD4+ T cells. J. Exp. Med. 178, 1655–1663 (1993).
Hofer, M. J. et al. Mice deficient in STAT1 but not STAT2 or IRF9 develop a lethal CD4+ T-cell-mediated disease following infection with lymphocytic choriomeningitis virus. J. Virol. 86, 6932–6946 (2012).
Lazear, H. M., Pinto, A. K., Vogt, M. R., Gale, M. Jr & Diamond, M. S. β-Interferon controls West Nile virus infection and pathogenesis in mice. J. Virol. 85, 7186–7194 (2011).
Shiow, L. R. et al. CD69 acts downstream of interferon-α/β to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440, 540–544 (2006).
Petricoin, E. F. et al. Antiproliferative action of interferon-α requires components of T-cell-receptor signalling. Nature 390, 629–632 (1997).
Kaser, A., Nagata, S. & Tilg, H. Interferon α augments activation-induced T cell death by upregulation of Fas (CD95/APO-1) and Fas ligand expression. Cytokine 11, 736–743 (1999).
Marshall, H. D., Urban, S. L. & Welsh, R. M. Virus-induced transient immune suppression and the inhibition of T cell proliferation by type I interferon. J. Virol. 85, 5929–5939 (2011).
Bromberg, J. F., Horvath, C. M., Wen, Z., Schreiber, R. D. & Darnell, J. E. Jr. Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon α and interferon γ. Proc. Natl. Acad. Sci. USA 93, 7673–7678 (1996).
Lee, C. K., Smith, E., Gimeno, R., Gertner, R. & Levy, D. E. STAT1 affects lymphocyte survival and proliferation partially independent of its role downstream of IFN-γ. J. Immunol. 164, 1286–1292 (2000).
Tanabe, Y. et al. Role of STAT1, STAT3, and STAT5 in IFN-α/β responses in T lymphocytes. J. Immunol. 174, 609–613 (2005).
Marrack, P., Kappler, J. & Mitchell, T. Type I interferons keep activated T cells alive. J. Exp. Med. 189, 521–530 (1999).
Aichele, P. et al. CD8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J. Immunol. 176, 4525–4529 (2006).
Kolumam, G. A., Thomas, S., Thompson, L. J., Sprent, J. & Murali-Krishna, K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202, 637–650 (2005).
Curtsinger, J. M., Valenzuela, J. O., Agarwal, P., Lins, D. & Mescher, M. F. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J. Immunol. 174, 4465–4469 (2005).
Keppler, S. J., Rosenits, K., Koegl, T., Vucikuja, S. & Aichele, P. Signal 3 cytokines as modulators of primary immune responses during infections: the interplay of type I IFN and IL-12 in CD8 T cell responses. PLoS ONE 7, e40865 (2012).
Gimeno, R., Lee, C. K., Schindler, C. & Levy, D. E. Stat1 and Stat2 but not Stat3 arbitrate contradictory growth signals elicited by α/β interferon in T lymphocytes. Mol. Cell. Biol. 25, 5456–5465 (2005).
Gil, M. P., Salomon, R., Louten, J. & Biron, C. A. Modulation of STAT1 protein levels: a mechanism shaping CD8 T-cell responses in vivo. Blood 107, 987–993 (2006).
Agarwal, P. et al. Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J. Immunol. 183, 1695–1704 (2009).
Marshall, H. D., Prince, A. L., Berg, L. J. & Welsh, R. M. IFN-α/β and self-MHC divert CD8 T cells into a distinct differentiation pathway characterized by rapid acquisition of effector functions. J. Immunol. 185, 1419–1428 (2010).
Cousens, L. P. et al. Two roads diverged: interferon α/β- and interleukin 12-mediated pathways in promoting T cell interferon γ responses during viral infection. J. Exp. Med. 189, 1315–1328 (1999).
Nguyen, K. B. et al. Critical role for STAT4 activation by type 1 interferons in the interferon-γ response to viral infection. Science 297, 2063–2066 (2002).
Nguyen, K. B. et al. Interferon α/β-mediated inhibition and promotion of interferon γ: STAT1 resolves a paradox. Nature Immunol. 1, 70–76 (2000).
Thompson, L. J., Kolumam, G. A., Thomas, S. & Murali-Krishna, K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J. Immunol. 177, 1746–1754 (2006).
Pinto, A. K. et al. A temporal role of type I interferon signaling in CD8+ T cell maturation during acute West Nile virus infection. PLoS Pathog. 7, e1002407 (2011).
Ramos, H. J. et al. Reciprocal responsiveness to interleukin-12 and interferon-α specifies human CD8+ effector versus central memory T-cell fates. Blood 113, 5516–5525 (2009).
Kohlmeier, J. E., Cookenham, T., Roberts, A. D., Miller, S. C. & Woodland, D. L. Type I interferons regulate cytolytic activity of memory CD8+ T cells in the lung airways during respiratory virus challenge. Immunity 33, 96–105 (2010).
Sung, J. H. et al. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell 150, 1249–1263 (2012).
Soudja, S. M., Ruiz, A. L., Marie, J. C. & Lauvau, G. Inflammatory monocytes activate memory CD8+ T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity 37, 549–562 (2012).
Crouse, J. et al. Type I interferons protect T cells against NK cell attack mediated by the activating receptor NCR1. Immunity 40, 961–973 (2014).
Xu, H. C. et al. Type I interferon protects antiviral CD8+ T cells from NK cell cytotoxicity. Immunity 40, 949–960 (2014).
Hwang, I. et al. Activation mechanisms of natural killer cells during influenza virus infection. PLoS ONE 7, e51858 (2012).
Martinez, J., Huang, X. & Yang, Y. Direct action of type I IFN on NK cells is required for their activation in response to vaccinia viral infection in vivo. J. Immunol. 180, 1592–1597 (2008).
Nguyen, K. B. et al. Coordinated and distinct roles for IFN-α/β, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 169, 4279–4287 (2002).
Lucas, M., Schachterle, W., Oberle, K., Aichele, P. & Diefenbach, A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity 26, 503–517 (2007).
Sun, J. C., Ma, A. & Lanier, L. L. IL-15-independent NK cell response to mouse cytomegalovirus infection. J. Immunol. 183, 2911–2914 (2009).
Baranek, T. et al. Differential responses of immune cells to type I interferon contribute to host resistance to viral infection. Cell Host Microbe 12, 571–584 (2012).
Miyagi, T. et al. High basal STAT4 balanced by STAT1 induction to control type 1 interferon effects in natural killer cells. J. Exp. Med. 204, 2383–2396 (2007).
Mack, E. A., Kallal, L. E., Demers, D. A. & Biron, C. A. Type 1 interferon induction of natural killer cell γ interferon production for defense during lymphocytic choriomeningitis virus infection. MBio 2, e00169-11 (2011).
Wang, J., Lin, Q., Langston, H. & Cooper, M. D. Resident bone marrow macrophages produce type 1 interferons that can selectively inhibit interleukin-7-driven growth of B lineage cells. Immunity 3, 475–484 (1995).
Lin, Q., Dong, C. & Cooper, M. D. Impairment of T and B cell development by treatment with a type I interferon. J. Exp. Med. 187, 79–87 (1998).
Bosio, E., Cluning, C. L. & Beilharz, M. W. Low-dose orally administered type I interferon reduces splenic B cell numbers in mice. J. Interferon Cytokine Res. 21, 721–728 (2001).
Le Bon, A. et al. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14, 461–470 (2001).
Swanson, C. L. et al. Type I IFN enhances follicular B cell contribution to the T cell-independent antibody response. J. Exp. Med. 207, 1485–1500 (2010).
Coro, E. S., Chang, W. L. & Baumgarth, N. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J. Immunol. 176, 4343–4351 (2006).
Chang, W. L. et al. Influenza virus infection causes global respiratory tract B cell response modulation via innate immune signals. J. Immunol. 178, 1457–1467 (2007).
Rau, F. C., Dieter, J., Luo, Z., Priest, S. O. & Baumgarth, N. B7-1/2 (CD80/CD86) direct signaling to B cells enhances IgG secretion. J. Immunol. 183, 7661–7671 (2009).
Heer, A. K. et al. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J. Immunol. 178, 2182–2191 (2007).
Fink, K. et al. Early type I interferon-mediated signals on B cells specifically enhance antiviral humoral responses. Eur. J. Immunol. 36, 2094–2105 (2006).
Bach, P. et al. Vesicular stomatitis virus glycoprotein displaying retrovirus-like particles induce a type I IFN receptor-dependent switch to neutralizing IgG antibodies. J. Immunol. 178, 5839–5847 (2007).
Purtha, W. E., Chachu, K. A., Virgin, H. W. & Diamond, M. S. Early B-cell activation after West Nile virus infection requires α/β interferon but not antigen receptor signaling. J. Virol. 82, 10964–10974 (2008).
Moseman, E. A. et al. B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity. Immunity 36, 415–426 (2012).
Biron, C. A. Interferons α and β as immune regulators — a new look. Immunity 14, 661–664 (2001).
Davidson, S., Crotta, S., McCabe, T. M. & Wack, A. Pathogenic potential of interferon αβ in acute influenza infection. Nature Commun. 5, 3864 (2014). This seminal publication shows that, in contrast to the dogma, type I IFNs can cause morbidity and mortality, as opposed to protection, during influenza virus infection.
Mandl, J. N. et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nature Med. 14, 1077–1087 (2008).
Jacquelin, B. et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J. Clin. Invest. 119, 3544–3555 (2009).
Rotger, M. et al. Comparative transcriptomics of extreme phenotypes of human HIV-1 infection and SIV infection in sooty mangabey and rhesus macaque. J. Clin. Invest. 121, 2391–2400 (2011).
McNally, J. M. et al. Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses. J. Virol. 75, 5965–5976 (2001).
Chi, B. et al. α and λ interferon together mediate suppression of CD4 T cells induced by respiratory syncytial virus. J. Virol. 80, 5032–5040 (2006).
Gil, M. P. et al. Regulating type 1 IFN effects in CD8 T cells during viral infections: changing STAT4 and STAT1 expression for function. Blood 120, 3718–3728 (2012).
Herbeuval, J. P. et al. Differential expression of IFN-α and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc. Natl Acad. Sci. USA 103, 7000–7005 (2006).
Hardy, A. W., Graham, D. R., Shearer, G. M. & Herbeuval, J. P. HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-α. Proc. Natl Acad. Sci. USA 104, 17453–17458 (2007).
Herbeuval, J. P. et al. CD4+ T-cell death induced by infectious and noninfectious HIV-1: role of type 1 interferon-dependent, TRAIL/DR5-mediated apoptosis. Blood 106, 3524–3531 (2005).
van Grevenynghe, J. et al. Loss of memory B cells during chronic HIV infection is driven by Foxo3a- and TRAIL-mediated apoptosis. J. Clin. Invest. 121, 3877–3888 (2011).
Liedtke, C., Groger, N., Manns, M. P. & Trautwein, C. Interferon-α enhances TRAIL-mediated apoptosis by up-regulating caspase-8 transcription in human hepatoma cells. J. Hepatol. 44, 342–349 (2006).
Shigeno, M. et al. Interferon-α sensitizes human hepatoma cells to TRAIL-induced apoptosis through DR5 upregulation and NF-κB inactivation. Oncogene 22, 1653–1662 (2003).
Toomey, N. L. et al. Induction of a TRAIL-mediated suicide program by interferon α in primary effusion lymphoma. Oncogene 20, 7029–7040 (2001).
Teijaro, J. R. et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340, 207–211 (2013).
Wilson, E. B. et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340, 202–207 (2013). References 128 and 129 were the first to show that type I IFNs contribute to pathogenesis by inducing suppressive mechanisms in chronic LCMV infection.
Herold, S. et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J. Exp. Med. 205, 3065–3077 (2008).
Hogner, K. et al. Macrophage-expressed IFN-β contributes to apoptotic alveolar epithelial cell injury in severe influenza virus pneumonia. PLoS Pathog. 9, e1003188 (2013).
Chaperot, L. et al. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J. Immunol. 176, 248–255 (2006).
Fujikura, D. et al. Type-I interferon is critical for FasL expression on lung cells to determine the severity of influenza. PLoS ONE 8, e55321 (2013).
McNally, B., Ye, F., Willette, M. & Flano, E. Local blockade of epithelial PDL-1 in the airways enhances T cell function and viral clearance during influenza virus infection. J. Virol. 87, 12916–12924 (2013).
Brincks, E. L. et al. The magnitude of the T cell response to a clinically significant dose of influenza virus is regulated by TRAIL. J. Immunol. 187, 4581–4588 (2011).
MacMicking, J. D. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nature Rev. Immunol. 12, 367–382 (2012).
Kazar, J., Gillmore, J. D. & Gordon, F. B. Effect of interferon and interferon inducers on infections with a nonviral intracellular microorganism, Chlamydia trachomatis. Infect. Immun. 3, 825–832 (1971).
de la Maza, L. M., Peterson, E. M., Goebel, J. M., Fennie, C. W. & Czarniecki, C. W. Interferon-induced inhibition of Chlamydia trachomatis: dissociation from antiviral and antiproliferative effects. Infect. Immun. 47, 719–722 (1985).
Ishihara, T. et al. Inhibition of Chlamydia trachomatis growth by human interferon-α: mechanisms and synergistic effect with interferon-γ and tumor necrosis factor-α. Biomed. Res. 26, 179–185 (2005).
Rothfuchs, A. G. et al. IFN-α/β-dependent, IFN-γ secretion by bone marrow-derived macrophages controls an intracellular bacterial infection. J. Immunol. 167, 6453–6461 (2001).
Rothfuchs, A. G. et al. STAT1 regulates IFN-αβ- and IFN-γ-dependent control of infection with Chlamydia pneumoniae by nonhemopoietic cells. J. Immunol. 176, 6982–6990 (2006).
Qiu, H. et al. Type I IFNs enhance susceptibility to Chlamydia muridarum lung infection by enhancing apoptosis of local macrophages. J. Immunol. 181, 2092–2102 (2008).
Opitz, B. et al. Legionella pneumophila induces IFNβ in lung epithelial cells via IPS-1 and IRF3, which also control bacterial replication. J. Biol. Chem. 281, 36173–36179 (2006).
Plumlee, C. R. et al. Interferons direct an effective innate response to Legionella pneumophila infection. J. Biol. Chem. 284, 30058–30066 (2009).
Schiavoni, G. et al. Type I IFN protects permissive macrophages from Legionella pneumophila infection through an IFN-γ-independent pathway. J. Immunol. 173, 1266–1275 (2004).
Gold, J. A. et al. Exogenous γ and α/β interferon rescues human macrophages from cell death induced by Bacillus anthracis. Infect. Immun. 72, 1291–1297 (2004).
Bukholm, G., Berdal, B. P., Haug, C. & Degre, M. Mouse fibroblast interferon modifies Salmonella typhimurium infection in infant mice. Infect. Immun. 45, 62–66 (1984).
Niesel, D. W., Hess, C. B., Cho, Y. J., Klimpel, K. D. & Klimpel, G. R. Natural and recombinant interferons inhibit epithelial cell invasion by Shigella spp. Infect. Immun. 52, 828–833 (1986).
Mancuso, G. et al. Type I IFN signaling is crucial for host resistance against different species of pathogenic bacteria. J. Immunol. 178, 3126–3133 (2007).
Parker, D. et al. Streptococcus pneumoniae DNA initiates type I interferon signaling in the respiratory tract. MBio 2, e00016-11 (2011).
Weigent, D. A., Huff, T. L., Peterson, J. W., Stanton, G. J. & Baron, S. Role of interferon in streptococcal infection in the mouse. Microb. Pathog. 1, 399–407 (1986).
Kelly-Scumpia, K. M. et al. Type I interferon signaling in hematopoietic cells is required for survival in mouse polymicrobial sepsis by regulating CXCL10. J. Exp. Med. 207, 319–326 (2010).
Weighardt, H. et al. Type I IFN modulates host defense and late hyperinflammation in septic peritonitis. J. Immunol. 177, 5623–5630 (2006).
Freudenberg, M. A. et al. A murine, IL-12-independent pathway of IFN-γ induction by Gram-negative bacteria based on STAT4 activation by type I IFN and IL-18 signaling. J. Immunol. 169, 1665–1668 (2002).
Auerbuch, V., Brockstedt, D. G., Meyer-Morse, N., O'Riordan, M. & Portnoy, D. A. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 200, 527–533 (2004).
Carrero, J. A., Calderon, B. & Unanue, E. R. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J. Exp. Med. 200, 535–540 (2004).
O'Connell, R. M. et al. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J. Exp. Med. 200, 437–445 (2004). References 155–157 were the first publications demonstrating an adverse effect of type I IFNs in intracellular infection with the bacterium L. monocytogenes.
Carrero, J. A., Calderon, B. & Unanue, E. R. Lymphocytes are detrimental during the early innate immune response against Listeria monocytogenes. J. Exp. Med. 203, 933–940 (2006).
Stockinger, S. et al. Production of type I IFN sensitizes macrophages to cell death induced by Listeria monocytogenes. J. Immunol. 169, 6522–6529 (2002).
Zwaferink, H., Stockinger, S., Hazemi, P., Lemmens-Gruber, R. & Decker, T. IFN-β increases listeriolysin O-induced membrane permeabilization and death of macrophages. J. Immunol. 180, 4116–4123 (2008).
Zwaferink, H., Stockinger, S., Reipert, S. & Decker, T. Stimulation of inducible nitric oxide synthase expression by β interferon increases necrotic death of macrophages upon Listeria monocytogenes infection. Infect. Immun. 76, 1649–1656 (2008).
Dresing, P., Borkens, S., Kocur, M., Kropp, S. & Scheu, S. A fluorescence reporter model defines “Tip-DCs” as the cellular source of interferon β in murine listeriosis. PLoS ONE 5, e15567 (2010).
Stockinger, S. et al. Characterization of the interferon-producing cell in mice infected with Listeria monocytogenes. PLoS Pathog. 5, e1000355 (2009).
Rayamajhi, M., Humann, J., Penheiter, K., Andreasen, K. & Lenz, L. L. Induction of IFN-α/β enables Listeria monocytogenes to suppress macrophage activation by IFN-γ. J. Exp. Med. 207, 327–337 (2010).
Kearney, S. J. et al. Type I IFNs downregulate myeloid cell IFN-γ receptor by inducing recruitment of an early growth response 3/NGFI-A binding protein 1 complex that silences ifngr1 transcription. J. Immunol. 191, 3384–3392 (2013).
Manca, C. et al. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J. Interferon Cytokine Res. 25, 694–701 (2005).
Ordway, D. et al. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J. Immunol. 179, 522–531 (2007).
Stanley, S. A., Johndrow, J. E., Manzanillo, P. & Cox, J. S. The type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J. Immunol. 178, 3143–3152 (2007).
Manca, C. et al. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-α/β. Proc. Natl Acad. Sci. USA 98, 5752–5757 (2001). This study was the first demonstration of type I IFNs contributing to the exacerbation of tuberculosis in experimental mouse models.
Cooper, A. M., Pearl, J. E., Brooks, J. V., Ehlers, S. & Orme, I. M. Expression of the nitric oxide synthase 2 gene is not essential for early control of Mycobacterium tuberculosis in the murine lung. Infect. Immun. 68, 6879–6882 (2000).
Berry, M. P. et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466, 973–977 (2010). This study provided the first evidence that type I IFN-mediated signalling is associated with active tuberculosis in humans.
Cliff, J. M. et al. Distinct phases of blood gene expression pattern through tuberculosis treatment reflect modulation of the humoral immune response. J. Infect. Dis. 207, 18–29 (2013).
Maertzdorf, J. et al. Human gene expression profiles of susceptibility and resistance in tuberculosis. Genes Immun. 12, 15–22 (2011).
Ottenhoff, T. H. et al. Genome-wide expression profiling identifies type 1 interferon response pathways in active tuberculosis. PLoS ONE 7, e45839 (2012).
Antonelli, L. R. et al. Intranasal poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J. Clin. Invest. 120, 1674–1682 (2010).
Mayer-Barber, K. D. et al. Innate and adaptive interferons suppress IL-1α and IL-1β production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity 35, 1023–1034 (2011).
McNab, F. W. et al. TPL-2-ERK1/2 signaling promotes host resistance against intracellular bacterial infection by negative regulation of type I IFN production. J. Immunol. 191, 1732–1743 (2013).
Redford, P. S. et al. Influenza A virus impairs control of Mycobacterium tuberculosis coinfection through a type I interferon receptor-dependent pathway. J. Infect. Dis. 209, 270–274 (2014).
Mayer-Barber, K. D. et al. Caspase-1 independent IL-1β production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 184, 3326–3330 (2010).
de Paus, R. A. et al. Inhibition of the type I immune responses of human monocytes by IFN-α and IFN-β. Cytokine 61, 645–655 (2013).
Novikov, A. et al. Mycobacterium tuberculosis triggers host type I IFN signaling to regulate IL-1β production in human macrophages. J. Immunol. 187, 2540–2547 (2011).
McNab, F. W. et al. Type I IFN induces IL-10 production in an IL-27-independent manner and blocks responsiveness to IFN-γ for production of IL-12 and bacterial killing in Mycobacterium tuberculosis-infected macrophages. J. Immunol. 193, 3600–3612 (2014). This key study demonstrates the mechanisms underlying the adverse effects of type I IFNs in tuberculosis, including blocking of the protective type II IFN action, as well as blocking of IL-12, IL-1 and TNF production, in part through IL-10 induction.
Guarda, G. et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34, 213–223 (2011). This was the first study to show inhibition of the inflammasome by type I IFNs.
Mayer-Barber, K. D. et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 511, 99–103 (2014). This seminal study shows the counter-regulatory function of IL-1 and type I IFNs in controlling the outcome of M. tuberculosis infection via eicosanoids.
Xu, X. J., Reichner, J. S., Mastrofrancesco, B., Henry, W. L. Jr & Albina, J. E. Prostaglandin E2 suppresses lipopolysaccharide-stimulated IFN-β production. J. Immunol. 180, 2125–2131 (2008). This study provided the first demonstration that prostaglandin E2 suppresses lipopolysaccharide-stimulated IFNβ production.
Coulombe, F. et al. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity 40, 554–568 (2014).
Teles, R. M. et al. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science 339, 1448–1453 (2013). In this key study, a mechanism is reported for type I IFN-mediated blocking of the protective role of type II IFN in tuberculosis.
Desvignes, L., Wolf, A. J. & Ernst, J. D. Dynamic roles of type I and type II IFNs in early infection with Mycobacterium tuberculosis. J. Immunol. 188, 6205–6215 (2012). This important study shows that type I IFNs contribute to protection against M. tuberculosis when type II IFN-mediated signalling is aberrant.
Bogunovic, D. et al. Mycobacterial disease and impaired IFN-γ immunity in humans with inherited ISG15 deficiency. Science 337, 1684–1688 (2012).
Mariotti, S. et al. Mycobacterium tuberculosis diverts α interferon-induced monocyte differentiation from dendritic cells into immunoprivileged macrophage-like host cells. Infect. Immun. 72, 4385–4392 (2004).
Fernandes-Alnemri, T. et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nature Immunol. 11, 385–393 (2010).
Henry, T., Brotcke, A., Weiss, D. S., Thompson, L. J. & Monack, D. M. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J. Exp. Med. 204, 987–994 (2007).
Henry, T. et al. Type I IFN signaling constrains IL-17A/F secretion by γδ T cells during bacterial infections. J. Immunol. 184, 3755–3767 (2010).
Shah, S. et al. Mycobacterium tuberculosis but not nonvirulent mycobacteria inhibits IFN-β and AIM2 inflammasome-dependent IL-1β production via its ESX-1 secretion system. J. Immunol. 191, 3514–3518 (2013).
Al Moussawi, K. et al. Type I interferon induction is detrimental during infection with the Whipple's disease bacterium, Tropheryma whipplei. PLoS Pathog. 6, e1000722 (2010).
de Almeida, L. A. et al. MyD88 and STING signaling pathways are required for IRF3-mediated IFN-β induction in response to Brucella abortus infection. PLoS ONE 6, e23135 (2011).
Patel, A. A., Lee-Lewis, H., Hughes-Hanks, J., Lewis, C. A. & Anderson, D. M. Opposing roles for interferon regulatory factor-3 (IRF-3) and type I interferon signaling during plague. PLoS Pathog. 8, e1002817 (2012).
Robinson, N. et al. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nature Immunol. 13, 954–962 (2012).
Rathinam, V. A. et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by Gram-negative bacteria. Cell 150, 606–619 (2012).
Broz, P. et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490, 288–291 (2012).
Martin, F. J. et al. Staphylococcus aureus activates type I IFN signaling in mice and humans through the Xr repeated sequences of protein A. J. Clin. Invest. 119, 1931–1939 (2009).
Diefenbach, A. et al. Type 1 interferon (IFNα/β) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity 8, 77–87 (1998).
Mattner, J. et al. Regulation of type 2 nitric oxide synthase by type 1 interferons in macrophages infected with Leishmania major. Eur. J. Immunol. 30, 2257–2267 (2000).
Mattner, J. et al. Protection against progressive leishmaniasis by IFN-β. J. Immunol. 172, 7574–7582 (2004).
Khouri, R. et al. IFN-β impairs superoxide-dependent parasite killing in human macrophages: evidence for a deleterious role of SOD1 in cutaneous leishmaniasis. J. Immunol. 182, 2525–2531 (2009).
Xin, L. et al. Type I IFN receptor regulates neutrophil functions and innate immunity to Leishmania parasites. J. Immunol. 184, 7047–7056 (2010).
Haque, A. et al. Type I interferons suppress CD4+ T-cell-dependent parasite control during blood-stage Plasmodium infection. Eur. J. Immunol. 41, 2688–2698 (2011).
Vigario, A. M. et al. Inhibition of Plasmodium yoelii blood-stage malaria by interferon α through the inhibition of the production of its target cell, the reticulocyte. Blood 97, 3966–3971 (2001).
Vigario, A. M. et al. Recombinant human IFN-α inhibits cerebral malaria and reduces parasite burden in mice. J. Immunol. 178, 6416–6425 (2007).
Voisine, C., Mastelic, B., Sponaas, A. M. & Langhorne, J. Classical CD11c+ dendritic cells, not plasmacytoid dendritic cells, induce T cell responses to Plasmodium chabaudi malaria. Int. J. Parasitol. 40, 711–719 (2010).
Liehl, P. et al. Host-cell sensors for Plasmodium activate innate immunity against liver-stage infection. Nature Med. 20, 47–53 (2014).
Costa, V. M. et al. Type I IFNs stimulate nitric oxide production and resistance to Trypanosoma cruzi infection. J. Immunol. 177, 3193–3200 (2006).
Koga, R. et al. TLR-dependent induction of IFN-β mediates host defense against Trypanosoma cruzi. J. Immunol. 177, 7059–7066 (2006).
Lopez, R., Demick, K. P., Mansfield, J. M. & Paulnock, D. M. Type I IFNs play a role in early resistance, but subsequent susceptibility, to the African trypanosomes. J. Immunol. 181, 4908–4917 (2008).
Chessler, A. D., Caradonna, K. L., Da'dara, A. & Burleigh, B. A. Type I interferons increase host susceptibility to Trypanosoma cruzi infection. Infect. Immun. 79, 2112–2119 (2011).
Une, C., Andersson, J. & Orn, A. Role of IFN-α/β and IL-12 in the activation of natural killer cells and interferon-γ production during experimental infection with Trypanosoma cruzi. Clin. Exp. Immunol. 134, 195–201 (2003).
Biondo, C. et al. IFN-α/β signaling is required for polarization of cytokine responses toward a protective type 1 pattern during experimental cryptococcosis. J. Immunol. 181, 566–573 (2008).
Biondo, C. et al. Recognition of yeast nucleic acids triggers a host-protective type I interferon response. Eur. J. Immunol. 41, 1969–1979 (2011).
del Fresno, C. et al. Interferon-β production via Dectin-1-Syk-IRF5 signaling in dendritic cells is crucial for immunity to C. albicans. Immunity 38, 1176–1186 (2013).
Majer, O. et al. Type I interferons promote fatal immunopathology by regulating inflammatory monocytes and neutrophils during Candida infections. PLoS Pathog. 8, e1002811 (2012).
Bourgeois, C. et al. Conventional dendritic cells mount a type I IFN response against Candida spp. requiring novel phagosomal TLR7-mediated IFN-β signaling. J. Immunol. 186, 3104–3112 (2011).
Inglis, D. O., Berkes, C. A., Hocking Murray, D. R. & Sil, A. Conidia but not yeast cells of the fungal pathogen Histoplasma capsulatum trigger a type I interferon innate immune response in murine macrophages. Infect. Immun. 78, 3871–3882 (2010).
Liu, L. et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J. Exp. Med. 208, 1635–1648 (2011).
van de Veerdonk, F. L. et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N. Engl. J. Med. 365, 54–61 (2011).
Morens, D. M., Taubenberger, J. K. & Fauci, A. S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J. Infect. Dis. 198, 962–970 (2008).
Li, W., Moltedo, B. & Moran, T. M. Type I interferon induction during influenza virus infection increases susceptibility to secondary Streptococcus pneumoniae infection by negative regulation of γδ T cells. J. Virol. 86, 12304–12312 (2012).
Nakamura, S., Davis, K. M. & Weiser, J. N. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J. Clin. Invest. 121, 3657–3665 (2011).
Shahangian, A. et al. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J. Clin. Invest. 119, 1910–1920 (2009).
Tian, X. et al. Poly I:C enhances susceptibility to secondary pulmonary infections by Gram-positive bacteria. PLoS ONE 7, e41879 (2012).
Navarini, A. A. et al. Increased susceptibility to bacterial superinfection as a consequence of innate antiviral responses. Proc. Natl Acad. Sci. USA 103, 15535–15539 (2006).
Kim, Y. G. et al. Viral infection augments Nod1/2 signaling to potentiate lethality associated with secondary bacterial infections. Cell Host Microbe 9, 496–507 (2011).
Belkaid, Y. & Hand, T. W. Role of the microbiota in immunity and inflammation. Cell 157, 121–141 (2014).
Ganal, S. C. et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity 37, 171–186 (2012).
Abt, M. C. et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 37, 158–170 (2012).
Tschurtschenthaler, M. et al. Type I interferon signalling in the intestinal epithelium affects Paneth cells, microbial ecology and epithelial regeneration. Gut 63, 1921–1931 (2014).
Kawashima, T. et al. Double-stranded RNA of intestinal commensal but not pathogenic bacteria triggers production of protective interferon-β. Immunity 38, 1187–1197 (2013). This study shows that the microbiota contributes to the initial production of protective type I IFNs. References 235 and 236 collectively demonstrate a novel interplay between the microbiota, type I IFNs and consequent protection against pathogens.
Gough, D. J., Messina, N. L., Clarke, C. J., Johnstone, R. W. & Levy, D. E. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity 36, 166–174 (2012).
Acknowledgements
The authors' work was supported by the Medical Research Council, UK (grants U117565642 to A.O.G. and U117597139 to A.W.), the European Research Council (grant 294682-TB-PATH to A.O.G.) and the National Institute of Allergy and Infectious Diseases, US National Institutes of Health (grants to K.M.-B. and A.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Cytosolic GAMP synthase
-
(cGAS). A cytosolic DNA sensor that catalyses the production of the second messenger cyclic di-GMP-AMP (cGAMP) in response to DNA, which is then recognized by the sensor and signalling intermediate STING (stimulator of interferon genes), triggering type I interferon production.
- Plasmacytoid dendritic cells
-
(pDCs). Immature dendritic cells with a morphology that resembles that of plasma cells. On a per-cell basis, pDCs are the main producers of type I interferons in response to viral infections or Toll-like receptor stimulation.
- Ribavirin
-
A drug that interferes with RNA metabolism and blocks viral replication. Ribavirin is used in combination with interferon-α to treat hepatitis C virus infection.
- M1 macrophage
-
A pro-inflammatory, or 'classically activated', subset of macrophages that are characterized by phagocytic activity and the expression of particular pro-inflammatory cytokines (such as tumour necrosis factor) and inflammatory mediators (such as inducible nitric oxide synthase).
Rights and permissions
About this article
Cite this article
McNab, F., Mayer-Barber, K., Sher, A. et al. Type I interferons in infectious disease. Nat Rev Immunol 15, 87–103 (2015). https://doi.org/10.1038/nri3787
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3787
This article is cited by
-
Longitudinal analysis of influenza vaccination implicates regulation of RIG-I signaling by DNA methylation
Scientific Reports (2024)
-
LUBAC is required for RIG-I sensing of RNA viruses
Cell Death & Differentiation (2024)
-
Deeper look into viruses: replication intermediates do code!
Plant Cell Reports (2024)
-
Heart-specific NFAT5 knockout suppresses type I interferon signaling and aggravates coxsackievirus-induced myocarditis
Basic Research in Cardiology (2024)
-
Deciphering the similarities and disparities of molecular mechanisms behind respiratory epithelium response to HCoV-229E and SARS-CoV-2 and drug repurposing, a systems biology approach
DARU Journal of Pharmaceutical Sciences (2024)