Key Points
-
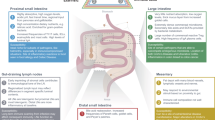
The intestine is essential for life, and it is continuously exposed to foreign antigens and other environmental agents. Therefore, it comprises the largest compartment of the immune system, with substantial amounts of organized lymphoid tissue and large populations of scattered innate and adaptive effector cells.
-
Many studies of intestinal immunology have overlooked the fact that the intestine comprises several anatomically defined segments that each have distinct physiological roles and immunological components.
-
The immune system of the small intestine focuses on protecting the ability of the surface epithelium to digest and absorb foodstuffs by defending it from infection. The mechanisms include: IL-17- and IL-22-producing T cells and innate lymphoid cells; the production of antimicrobial peptides; and intraepithelial T cells with innate and cytolytic effector functions. Regulatory T cells help to prevent hypersensitivity reactions to dietary antigens.
-
The large intestine (colon) is not involved in digestion but is the reservoir for huge numbers of commensal microorganisms that are essential for health. The colonic immune system recognizes these microorganisms as potential hazards and keeps them 'at arms length' without expelling them. This involves the production of a thick mucus layer, the generation of IgA antibodies and the presence of large numbers of regulatory T cells.
-
These different aspects of immune function are served by distinct kinds of secondary lymphoid organs and are shaped by factors in the local environment, such as dietary components and bacterial metabolites.
-
Elucidating the factors that determine the anatomical compartmentalization of the intestinal immune system will improve our understanding of why intestinal diseases tend to occur at particular anatomical sites in the intestine.
Abstract
The intestine represents the largest compartment of the immune system. It is continually exposed to antigens and immunomodulatory agents from the diet and the commensal microbiota, and it is the port of entry for many clinically important pathogens. Intestinal immune processes are also increasingly implicated in controlling disease development elsewhere in the body. In this Review, we detail the anatomical and physiological distinctions that are observed in the small and large intestines, and we suggest how these may account for the diversity in the immune apparatus that is seen throughout the intestine. We describe how the distribution of innate, adaptive and innate-like immune cells varies in different segments of the intestine and discuss the environmental factors that may influence this. Finally, we consider the implications of regional immune specialization for inflammatory disease in the intestine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ouellette, A. J. Paneth cells and innate mucosal immunity. Curr. Opin. Gastroenterol. 26, 547–553 (2010).
Clevers, H. C. & Bevins, C. L. Paneth cells: maestros of the small intestinal crypts. Annu. Rev. Physiol. 75, 289–311 (2013). This review covers the physiology and functions of Paneth cells.
Sato, T. et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 (2011).
Adolph, T. E. et al. Paneth cells as a site of origin for intestinal inflammation. Nature 503, 272–276 (2013).
Cadwell, K. et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456, 259–263 (2008).
Gunther, C. et al. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature 477, 335–339 (2011).
Wittkopf, N. et al. Lack of intestinal epithelial Atg7 affects Paneth cell granule formation but does not compromise immune homeostasis in the gut. Clin. Dev. Immunol. 2012, 278059 (2012).
Kaser, A. & Blumberg, R. S. Autophagy, microbial sensing, endoplasmic reticulum stress, and epithelial function in inflammatory bowel disease. Gastroenterology 140, 1738–1747 (2011). This is an accessible review which places the intestinal epithelial cell in an immunological and anatomical context.
Vandussen, K. L. et al. Genetic variants synthesize to produce Paneth cell phenotypes that define subtypes of Crohn's disease. Gastroenterology 146, 200–209 (2014).
Liu, B. et al. Irgm1-deficient mice exhibit Paneth cell abnormalities and increased susceptibility to acute intestinal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G573–G584 (2013).
Johansson, M. E. et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl Acad. Sci. USA 105, 15064–15069 (2008). This study describes the structure and properties of the mucus layer in different parts of the intestine.
Hansson, G. C. Role of mucus layers in gut infection and inflammation. Curr. Opin. Microbiol. 15, 57–62 (2012).
Bancroft, A. J., McKenzie, A. N. & Grencis, R. K. A critical role for IL-13 in resistance to intestinal nematode infection. J. Immunol. 160, 3453–3461 (1998).
Steenwinckel, V. et al. IL-9 promotes IL-13-dependent Paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa. J. Immunol. 182, 4737–4743 (2009).
Klose, C. S. et al. A T-bet gradient controls the fate and function of CCR6−RORγt+ innate lymphoid cells. Nature 494, 261–265 (2013).
Johansson, M. E. et al. Composition and functional role of the mucus layers in the intestine. Cell. Mol. Life Sci. 68, 3635–3641 (2011).
Velcich, A. et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 295, 1726–1729 (2002).
Van der Sluis, M. et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131, 117–129 (2006).
Zaph, C. et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature 446, 552–556 (2007).
Rescigno, M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 32, 256–264 (2011).
Maldonado-Contreras, A. L. & McCormick, B. A. Intestinal epithelial cells and their role in innate mucosal immunity. Cell Tissue Res. 343, 5–12 (2011).
Wang, Y. et al. Regional mucosa-associated microbiota determine physiological expression of TLR2 and TLR4 in murine colon. PLoS ONE 5, e13607 (2010).
Ortega-Cava, C. F. et al. Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J. Immunol. 170, 3977–3985 (2003).
Lala, S. et al. Crohn's disease and the NOD2 gene: a role for paneth cells. Gastroenterology 125, 47–57 (2003).
Barrett, J. C. et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nature Genet. 40, 955–962 (2008).
Hugot, J. P. et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411, 599–603 (2001).
Mabbott, N. A., Donaldson, D. S., Ohno, H., Williams, I. R. & Mahajan, A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 6, 666–677 (2013). This review describes the location, functions and development of M cells in different sites.
Cornes, J. S. Number, size, and distribution of Peyer's patches in the human small intestine: Part I The development of Peyer's patches. Gut 6, 225–229 (1965).
Owen, R. L., Piazza, A. J. & Ermak, T. H. Ultrastructural and cytoarchitectural features of lymphoreticular organs in the colon and rectum of adult BALB/c mice. Am. J. Anat. 190, 10–18 (1991).
Perry, G. A. & Sharp, J. G. Characterization of proximal colonic lymphoid tissue in the mouse. Anat. Rec. 220, 305–312 (1988).
Lee, A. Y. et al. Dendritic cells in colonic patches and iliac lymph nodes are essential in mucosal IgA induction following intrarectal administration via CCR7 interaction. Eur. J. Immunol. 38, 1127–1137 (2008).
Masahata, K. et al. Generation of colonic IgA-secreting cells in the caecal patch. Nature Commun. 5, 3704 (2014). This novel study indicates that the recognition of the intestinal microbiota in the Peyer's patches and caecal patches leads to the generation of IgA-producing plasma cells in the small intestine and colon, respectively.
Baptista, A. P. et al. Colonic patch and colonic SILT development are independent and differentially regulated events. Mucosal Immunol. 6, 511–521 (2013).
Herbrand, H., Bernhardt, G., Forster, R. & Pabst, O. Dynamics and function of solitary intestinal lymphoid tissue. Crit. Rev. Immunol. 28, 1–13 (2008). This review discusses the ILFs that are found in different regions of the intestine.
Pabst, O. et al. Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur. J. Immunol. 35, 98–107 (2005).
Tsuji, M. et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity 29, 261–271 (2008).
Kanamori, Y. et al. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J. Exp. Med. 184, 1449–1459 (1996).
Bouskra, D. et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 456, 507–510 (2008).
Trepel, F. Number and distribution of lymphocytes in man. A critical analysis. Klin. Wochenschr. 52, 511–515 (1974).
Moghaddami, M., Cummins, A. & Mayrhofer, G. Lymphocyte-filled villi: comparison with other lymphoid aggregations in the mucosa of the human small intestine. Gastroenterology 115, 1414–1425 (1998).
O'Leary, A. D. & Sweeney, E. C. Lymphoglandular complexes of the colon: structure and distribution. Histopathology 10, 267–283 (1986).
Hamada, H. et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J. Immunol. 168, 57–64 (2002).
McDonald, K. G., McDonough, J. S., Dieckgraefe, B. K. & Newberry, R. D. Dendritic cells produce CXCL13 and participate in the development of murine small intestine lymphoid tissues. Am. J. Pathol. 176, 2367–2377 (2010).
Velaga, S. et al. Chemokine receptor CXCR5 supports solitary intestinal lymphoid tissue formation, B cell homing, and induction of intestinal IgA responses. J. Immunol. 182, 2610–2619 (2009).
Knoop, K. A., Butler, B. R., Kumar, N., Newberry, R. D. & Williams, I. R. Distinct developmental requirements for isolated lymphoid follicle formation in the small and large intestine: RANKL is essential only in the small intestine. Am. J. Pathol. 179, 1861–1871 (2011). This is one of the few studies that directly compares the development of secondary lymphoid organs in the small and large intestines.
Tilney, N. L. Patterns of lymphatic drainage in the adult laboratory rat. J. Anat. 109, 369–383 (1971).
Carter, P. B. & Collins, F. M. The route of enteric infection in normal mice. J. Exp. Med. 139, 1189–1203 (1974).
Van den Broeck, W., Derore, A. & Simoens, P. Anatomy and nomenclature of murine lymph nodes: Descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J. Immunol. Methods 312, 12–19 (2006).
Ferguson, A. Intraepithelial lymphocytes of the small intestine. Gut 18, 921–937 (1977).
Sheridan, B. S. & Lefrancois, L. Intraepithelial lymphocytes: to serve and protect. Curr. Gastroenterol. Rep. 12, 513–521 (2010).
Cheroutre, H., Lambolez, F. & Mucida, D. The light and dark sides of intestinal intraepithelial lymphocytes. Nature Rev. Immunol. 11, 445–456 (2011).
Hayday, A. & Gibbons, D. Brokering the peace: the origin of intestinal T cells. Mucosal Immunol. 1, 172–174 (2008). In this review, the concept of distinct ontogenic and functional subsets of IELs is defined.
Gangadharan, D. et al. Identification of pre- and postselection TCRαβ+ intraepithelial lymphocyte precursors in the thymus. Immunity 25, 631–641 (2006).
Pobezinsky, L. A. et al. Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nature Immunol. 13, 569–578 (2012).
Leishman, A. J. et al. Precursors of functional MHC class I- or class II-restricted CD8αα+ T cells are positively selected in the thymus by agonist self-peptides. Immunity 16, 355–364 (2002).
Beagley, K. W. et al. Differences in intraepithelial lymphocyte T cell subsets isolated from murine small versus large intestine. J. Immunol. 154, 5611–5619 (1995).
Boll, G., Rudolphi, A., Spiess, S. & Reimann, J. Regional specialization of intraepithelial T cells in the murine small and large intestine. Scand. J. Immunol. 41, 103–113 (1995).
Camerini, V., Panwala, C. & Kronenberg, M. Regional specialization of the mucosal immune system. Intraepithelial lymphocytes of the large intestine have a different phenotype and function than those of the small intestine. J. Immunol. 151, 1765–1776 (1993). This study is a direct comparison of intraepithelial lymphocytes in the small and large intestines.
Suzuki, K. et al. Gut cryptopatches: direct evidence of extrathymic anatomical sites for intestinal T lymphopoiesis. Immunity 13, 691–702 (2000).
Ibraghimov, A. R. & Lynch, R. G. Heterogeneity and biased T cell receptor alpha/beta repertoire of mucosal CD8+ cells from murine large intestine: implications for functional state. J. Exp. Med. 180, 433–444 (1994).
Bandeira, A. et al. Localization of γ/δ T cells to the intestinal epithelium is independent of normal microbial colonization. J. Exp. Med. 172, 239–244 (1990).
Suzuki, H., Jeong, K. I., Itoh, K. & Doi, K. Regional variations in the distributions of small intestinal intraepithelial lymphocytes in germ-free and specific pathogen-free mice. Exp. Mol. Pathol. 72, 230–235 (2002).
Jiang, W. et al. Recognition of gut microbiota by NOD2 is essential for the homeostasis of intestinal intraepithelial lymphocytes. J. Exp. Med. 210, 2465–2476 (2013).
Selby, W. S., Janossy, G. & Jewell, D. P. Immunohistological characterisation of intraepithelial lymphocytes of the human gastrointestinal tract. Gut 22, 169–176 (1981).
Lundqvist, C., Baranov, V., Hammarstrom, S., Athlin, L. & Hammarstrom, M. L. Intra-epithelial lymphocytes. Evidence for regional specialization and extrathymic T cell maturation in the human gut epithelium. Int. Immunol. 7, 1473–1487 (1995).
Jabri, B. & Ebert, E. Human CD8+ intraepithelial lymphocytes: a unique model to study the regulation of effector cytotoxic T lymphocytes in tissue. Immunol. Rev. 215, 202–214 (2007).
Ivanov, I. I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Maynard, C. L. et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nature Immunol. 8, 931–941 (2007).
Sathaliyawala, T. et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38, 187–197 (2013).
Veenbergen, S. & Samsom, J. N. Maintenance of small intestinal and colonic tolerance by IL-10-producing regulatory T cell subsets. Curr. Opin. Immunol. 24, 269–276 (2012). This is a review of the mechanisms that may control tolerance in the different segments of the intestine.
Denning, T. L. et al. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. J. Immunol. 187, 733–747 (2011). This detailed study describes gradients of DCs, macrophages, effector T cells and T Reg cells along the length of the intestine.
Wolff, M. J. et al. TH17, TH22 and TReg cells are enriched in the healthy human cecum. PLoS ONE 7, e41373 (2012).
Brandtzaeg, P. Function of mucosa-associated lymphoid tissue in antibody formation. Immunol. Invest. 39, 303–355 (2010).
Brandtzaeg, P., Carlsen, H. & Farstad, I. N. in Mucosal Immunology, 3rd edition (eds Mestecky, J. et al.) 617–665 (Academic Press, 2005).
Brandtzaeg, P. & Johansen, F. E. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol. Rev. 206, 32–63 (2005).
Smith, P. D., MacDonald, T. T. & Blumberg, R. S. in Principles of Mucosal Immunology (eds Smith, P. D., MacDonald, T. T. & Blumberg, R. S.) 103–119 (Garland Science, 2013).
Kett, K., Brandtzaeg, P., Radl, J. & Haaijman, J. J. Different subclass distribution of IgA-producing cells in human lymphoid organs and various secretory tissues. J. Immunol. 136, 3631–3635 (1986).
Crago, S. S. et al. Distribution of IgA1-, IgA2-, and J chain-containing cells in human tissues. J. Immunol. 132, 16–18 (1984).
He, B. et al. Intestinal bacteria trigger T cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26, 812–826 (2007).
Lin, M., Du, L., Brandtzaeg, P. & Pan-Hammarstrom, Q. IgA subclass switch recombination in human mucosal and systemic immune compartments. Mucosal Immunol. 7, 511–520 (2014). This study provides a definitive description of IgA isotype specialization in different segments of the intestine.
Kett, K. et al. Intestinal B-cell isotype response in relation to local bacterial load: evidence for immunoglobulin A subclass adaptation. Gastroenterology 109, 819–825 (1995).
Plaut, A. G., Wistar, R. Jr & Capra, J. D. Differential susceptibility of human IgA immunoglobulins to streptococcal IgA protease. J. Clin. Invest. 54, 1295–1300 (1974).
Kilian, M., Reinholdt, J., Lomholt, H., Poulsen, K. & Frandsen, E. V. Biological significance of IgA1 proteases in bacterial colonization and pathogenesis: critical evaluation of experimental evidence. APMIS 104, 321–338 (1996).
Bonner, A., Almogren, A., Furtado, P. B., Kerr, M. A. & Perkins, S. J. The nonplanar secretory IgA2 and near planar secretory IgA1 solution structures rationalize their different mucosal immune responses. J. Biol. Chem. 284, 5077–5087 (2009).
Tarkowski, A. et al. Immunization of humans with polysaccharide vaccines induces systemic, predominantly polymeric IgA2-subclass antibody responses. J. Immunol. 144, 3770–3778 (1990).
Pakkanen, S. H. et al. Expression of homing receptors on IgA1 and IgA2 plasmablasts in blood reflects differential distribution of IgA1 and IgA2 in various body fluids. Clin. Vaccine Immunol. 17, 393–401 (2010).
Spits, H. et al. Innate lymphoid cells — a proposal for uniform nomenclature. Nature Rev. Immunol. 13, 145–149 (2013). This is an important review which defines the current classification of ILCs.
Vonarbourg, C. et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt+ innate lymphocytes. Immunity 33, 736–751 (2010).
Nussbaum, J. C. et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502, 245–248 (2013).
Mjosberg, J. M. et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nature Immunol. 12, 1055–1062 (2011).
Sawa, S. et al. Lineage relationship analysis of RORγt+ innate lymphoid cells. Science 330, 665–669 (2010).
Chen, V. L., Surana, N. K., Duan, J. & Kasper, D. L. Role of murine intestinal interleukin-1 receptor 1-expressing lymphoid tissue inducer-like cells in Salmonella infection. PLoS ONE 8, e65405 (2013).
Tumanov, A. V. et al. Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe 10, 44–53 (2011).
Satoh-Takayama, N. et al. Lymphotoxin-β receptor-independent development of intestinal IL-22-producing NKp46+ innate lymphoid cells. Eur. J. Immunol. 41, 780–786 (2011).
Reynders, A. et al. Identity, regulation and in vivo function of gut NKp46+RORγt+ and NKp46+RORγt− lymphoid cells. EMBO J. 30, 2934–2947 (2011).
Lecuyer, E. et al. Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce gut IgA and specific T helper 17 cell responses. Immunity 40, 608–620 (2014). This very recent report describes how SFB interacts with secondary lymphoid tissues in the intestine to shape local immune cell functions.
Le Bourhis, L. et al. Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol. 32, 212–218 (2011).
Dunne, M. R. et al. Persistent changes in circulating and intestinal γδ T cell subsets, invariant natural killer T cells and mucosal-associated invariant T cells in children and adults with coeliac disease. PLoS ONE 8, e76008 (2013).
Gold, M. C. & Lewinsohn, D. M. Co-dependents: MR1-restricted MAIT cells and their antimicrobial function. Nature Rev. Microbiol. 11, 14–19 (2013).
Kjer-Nielsen, L. et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491, 717–723 (2012). This paper provides the first description of how the preferential location of MAIT cells reflects their recognition of microbial products.
Patel, O. et al. Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nature Commun. 4, 2142 (2013).
Reantragoon, R. et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J. Exp. Med. 210, 2305–2320 (2013).
Dusseaux, M. et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117, 1250–1259 (2011).
Le Bourhis, L. et al. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog. 9, e1003681 (2013).
Zeissig, S. & Blumberg, R. S. Commensal microbiota and NKT cells in the control of inflammatory diseases at mucosal surfaces. Curr. Opin. Immunol. 25, 690–696 (2013).
Wingender, G. & Kronenberg, M. Role of NKT cells in the digestive system. IV. The role of canonical natural killer T cells in mucosal immunity and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G1–G8 (2008).
Wingender, G. et al. Intestinal microbes affect phenotypes and functions of invariant natural killer T cells in mice. Gastroenterology 143, 418–428 (2012).
Olszak, T. et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336, 489–493 (2012).
Loh, L., Ivarsson, M. A., Michaelsson, J., Sandberg, J. K. & Nixon, D. F. Invariant natural killer T cells developing in the human fetus accumulate and mature in the small intestine. Mucosal Immunol. 7, 1233–1243 (2014).
An, D. et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 156, 123–133 (2014).
Persson, E. K., Jaensson, E. & Agace, W. W. The diverse ontogeny and function of murine small intestinal dendritic cell/macrophage subsets. Immunobiology 215, 692–697 (2010).
Cerovic, V., Bain, C. C., Mowat, A. M. & Milling, S. W. F. Intestinal macrophages and dendritic cells: what's the difference? Trends Immunol. 35, 270–277 (2014). This review highlights the distinct properties and ontogeny of intestinal DCs and macrophages.
Ueda, Y. et al. Commensal microbiota induce LPS hyporesponsiveness in colonic macrophages via the production of IL-10. Int. Immunol. 22, 953–962 (2010).
Melillo, J. A. et al. Dendritic cell (DC)-specific targeting reveals Stat3 as a negative regulator of DC function. J. Immunol. 184, 2638–2645 (2010).
Takeda, K. et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10, 39–49 (1999).
Murai, M. et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nature Immunol. 10, 1178–1184 (2009).
Hadis, U. et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 34, 237–246 (2011). This study defines the anatomical and mechanistic pathways that control the induction and secondary expansion of T Reg cells in the intestine.
Shaw, M. H., Kamada, N., Kim, Y. G. & Nunez, G. Microbiota-induced IL-1β, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J. Exp. Med. 209, 251–258 (2012).
Zigmond, E. et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity 40, 720–733 (2014).
Shouval, D. S. et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity 40, 706–719 (2014).
Bain, C. C. et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nature Immunol. http://dx.doi.org/10.1038/ni.2967 (2014).
Bain, C. C. & Mowat, A. M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 260, 102–117 (2014).
Hume, D. A., Perry, V. H. & Gordon, S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localisation of antigen F4/80: macrophages associated with epithelia. Anat. Rec. 210, 503–512 (1984).
Nagashima, R., Maeda, K., Imai, Y. & Takahashi, T. Lamina propria macrophages in the human gastrointestinal mucosa: their distribution, immunohistological phenotype, and function. J. Histochem. Cytochem. 44, 721–731 (1996).
Weber, B., Saurer, L. & Mueller, C. Intestinal macrophages: differentiation and involvement in intestinal immunopathologies. Semin. Immunopathol. 31, 171–184 (2009).
Ogino, T. et al. Increased Th17-inducing activity of CD14+ CD163low myeloid cells in intestinal lamina propria of patients with Crohn's disease. Gastroenterology 145, 1380–1391 (2013).
Hume, D. A., Allan, W., Hogan, P. G. & Doe, W. F. Immunohistochemical characterisation of macrophages in human liver and gastrointestinal tract: expression of CD4, HLA-DR, OKM1, and the mature macrophage marker 25F9 in normal and diseased tissue. J. Leukoc. Biol. 42, 474–484 (1987).
Mahida, Y. R., Patel, S., Gionchetti, P., Vaux, D. & Jewell, D. P. Macrophage subpopulations in lamina propria of normal and inflamed colon and terminal ileum. Gut 30, 826–834 (1989).
Rugtveit, J., Brandtzaeg, P., Halstensen, T. S., Fausa, O. & Scott, H. Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut 35, 669–674 (1994).
Carlsen, H. S., Yamanaka, T., Scott, H., Rugtveit, J. & Brandtzaeg, P. The proportion of CD40+ mucosal macrophages is increased in inflammatory bowel disease whereas CD40 ligand (CD154)+ T cells are relatively decreased, suggesting differential modulation of these costimulatory molecules in human gut lamina propria. Inflamm. Bowel Dis. 12, 1013–1024 (2006).
Devosse, T. et al. Formyl peptide receptor-like 2 is expressed and functional in plasmacytoid dendritic cells, tissue-specific macrophage subpopulations, and eosinophils. J. Immunol. 182, 4974–4984 (2009).
McElrath, M. J. et al. Comprehensive assessment of HIV target cells in the distal human gut suggests increasing HIV susceptibility toward the anus. J. Acquir. Immune Def. Syndr. 63, 263–271 (2013).
Rugtveit, J. et al. Respiratory burst of intestinal macrophages in inflammatory bowel disease is mainly caused by CD14+L1+ monocyte derived cells. Gut 37, 367–373 (1995).
Smythies, L. E. et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Invest. 115, 66–75 (2005).
Smythies, L. E. et al. Inflammation anergy in human intestinal macrophages is due to Smad-induced IκBα expression and NF-κB inactivation. J. Biol. Chem. 285, 19593–19604 (2010).
Smith, P. D. et al. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 4, 31–42 (2011).
Bogunovic, M. et al. Origin of the lamina propria dendritic cell network. Immunity 31, 513–525 (2009).
Schulz, O. et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J. Exp. Med. 206, 3101–3114 (2009).
Cerovic, V. et al. Intestinal CD103− dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol. 6, 104–113 (2013).
Persson, E. K., Scott, C. L., Mowat, A. M. & Agace, W. W. Dendritic cell subsets in the intestinal lamina propria: Ontogeny and function. Eur. J. Immunol. 43, 3098–3107 (2013). This review describes the different well-defined subsets of intestinal DCs and how their composition varies along the gastrointestinal tract.
Edelson, B. T. et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. J. Exp. Med. 207, 823–836 (2010).
Cerovic, V. et al. Lymph-borne CD8α+ dendritic cells are uniquely able to cross-prime CD8+ T cells with antigen acquired from intestinal epithelial cells. Mucosal Immunol. http://dx.doi.org/10.1038/mi.2014.40 (2014).
Schlitzer, A. et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 38, 970–983 (2013).
Persson, E. K. et al. IRF4 transcription-factor-dependent CD103+CD11b+ dendritic cells drive mucosal T helper 17 cell differentiation. Immunity 38, 958–969 (2013).
Poulin, L. F. et al. DNGR-1 is a specific and universal marker of mouse and human Batf3-dependent dendritic cells in lymphoid and nonlymphoid tissues. Blood 119, 6052–6062 (2012).
Watchmaker, P. B. et al. Comparative transcriptional and functional profiling defines conserved programs of intestinal DC differentiation in humans and mice. Nature Immunol. 15, 98–108 (2014).
Satpathy, A. T. et al. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nature Immunol. 14, 937–948 (2013).
Welty, N. E. et al. Intestinal lamina propria dendritic cells maintain T cell homeostasis but do not affect commensalism. J. Exp. Med. 210, 2011–2024 (2013).
Wendland, M. et al. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc. Natl Acad. Sci. USA 104, 6347–6352 (2007).
Baumgart, D. C. et al. Aberrant plasmacytoid dendritic cell distribution and function in patients with Crohn's disease and ulcerative colitis. Clin. Exp. Immunol. 166, 46–54 (2011).
Kwa, S. et al. Plasmacytoid dendritic cells are recruited to the colorectum and contribute to immune activation during pathogenic SIV infection in rhesus macaques. Blood 118, 2763–2773 (2011).
Smit, J. J. et al. The role of intestinal dendritic cells subsets in the establishment of food allergy. Clin. Exp. Allergy 41, 890–898 (2011).
Mizuno, S. et al. CCR9+ plasmacytoid dendritic cells in the small intestine suppress development of intestinal inflammation in mice. Immunol. Lett. 146, 64–69 (2012).
Karlis, J. et al. Characterization of colonic and mesenteric lymph node dendritic cell subpopulations in a murine adoptive transfer model of inflammatory bowel disease. Inflamm. Bowel Dis. 10, 834–847 (2004).
Yrlid, U. et al. Plasmacytoid dendritic cells do not migrate in intestinal or hepatic lymph. J. Immunol. 177, 6115–6121 (2006).
Dasgupta, S., Erturk-Hasdemir, D., Ochoa-Reparaz, J., Reinecker, H. C. & Kasper, D. L. Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe 15, 413–423 (2014).
Rothkotter, H. J., Kirchhoff, T. & Pabst, R. Lymphoid and non-lymphoid cells in the epithelium and lamina propria of intestinal mucosa of pigs. Gut 35, 1582–1589 (1994).
Bischoff, S. C. et al. Quantitative assessment of intestinal eosinophils and mast cells in inflammatory bowel disease. Histopathology 28, 1–13 (1996).
Rothenberg, M. E., Mishra, A., Brandt, E. B. & Hogan, S. P. Gastrointestinal eosinophils. Immunol. Rev. 179, 139–155 (2001).
Bain, C. C. et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 6, 498–510 (2013).
Bischoff, S. C. Physiological and pathophysiological functions of intestinal mast cells. Semin. Immunopathol. 31, 185–205 (2009).
Yu, L. C. & Perdue, M. H. Role of mast cells in intestinal mucosal function: studies in models of hypersensitivity and stress. Immunol. Rev. 179, 61–73 (2001).
Carlens, J. et al. Common γ-chain-dependent signals confer selective survival of eosinophils in the murine small intestine. J. Immunol. 183, 5600–5607 (2009).
Straumann, A. et al. Cytokine expression in healthy and inflamed mucosa: probing the role of eosinophils in the digestive tract. Inflamm. Bowel Dis. 11, 720–726 (2005).
Mishra, A., Hogan, S. P., Lee, J. J., Foster, P. S. & Rothenberg, M. E. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J. Clin. Invest. 103, 1719–1727 (1999).
Ahrens, R. et al. Intestinal macrophage/epithelial cell-derived CCL11/eotaxin-1 mediates eosinophil recruitment and function in pediatric ulcerative colitis. J. Immunol. 181, 7390–7399 (2008).
Lampinen, M., Waddell, A., Ahrens, R., Carlson, M. & Hogan, S. P. CD14+CD33+ myeloid cell–CCL11–eosinophil signature in ulcerative colitis. J. Leukoc. Biol. 94, 1061–1070 (2013).
Brandt, E. B. et al. The α4bβ7-integrin is dynamically expressed on murine eosinophils and involved in eosinophil trafficking to the intestine. Clin. Exp. Allergy 36, 543–553 (2006).
Wen, T. et al. The pan-B cell marker CD22 is expressed on gastrointestinal eosinophils and negatively regulates tissue eosinophilia. J. Immunol. 188, 1075–1082 (2012).
Chu, V. T. et al. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity 40, 582–593 (2014). This study provides novel information on the potential functions of intestinal eosinophils.
Dvorak, A. M., Monahan, R. A., Osage, J. E. & Dickersin, G. R. Crohn's disease: transmission electron microscopic studies. II. Immunologic inflammatory response. Alterations of mast cells, basophils, eosinophils, and the microvasculature. Hum. Pathol. 11, 606–619 (1980).
Strobel, S., Miller, H. R. & Ferguson, A. Human intestinal mucosal mast cells: evaluation of fixation and staining techniques. J. Clin. Pathol. 34, 851–858 (1981).
Nishida, Y. et al. Different distribution of mast cells and macrophages in colonic mucosa of patients with collagenous colitis and inflammatory bowel disease. Hepatogastroenterology 49, 678–682 (2002).
Lloyd, G., Green, F. H., Fox, H., Mani, V. & Turnberg, L. A. Mast cells and immunoglobulin E in inflammatory bowel disease. Gut 16, 861–865 (1975).
Miller, H. R. & Pemberton, A. D. Tissue-specific expression of mast cell granule serine proteinases and their role in inflammation in the lung and gut. Immunology 105, 375–390 (2002).
Miller, H. R. et al. Granule proteinases define mast cell heterogeneity in the serosa and the gastrointestinal mucosa of the mouse. Immunology 65, 559–566 (1988).
Kunkel, E. J. et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192, 761–768 (2000).
Pabst, O. et al. Chemokine receptor CCR9 contributes to the localization of plasma cells to the small intestine. J. Exp. Med. 199, 411–416 (2004).
Svensson, M. et al. CCL25 mediates the localization of recently activated CD8αβ+ lymphocytes to the small-intestinal mucosa. J. Clin. Invest. 110, 1113–1121 (2002).
Feng, N. et al. Redundant role of chemokines CCL25/TECK and CCL28/MEC in IgA+ plasmablast recruitment to the intestinal lamina propria after rotavirus infection. J. Immunol. 176, 5749–5759 (2006).
Hieshima, K. et al. CC chemokine ligands 25 and 28 play essential roles in intestinal extravasation of IgA antibody-secreting cells. J. Immunol. 173, 3668–3675 (2004).
Hu, S., Yang, K., Yang, J., Li, M. & Xiong, N. Critical roles of chemokine receptor CCR10 in regulating memory IgA responses in intestines. Proc. Natl Acad. Sci. USA 108, E1035–E1044 (2011).
Kim, S. V. et al. GPR15-mediated homing controls immune homeostasis in the large intestine mucosa. Science 340, 1456–1459 (2013). This paper describes a novel colon-specific chemoattractant.
Agace, W. W. & Persson, E. K. How vitamin A metabolizing dendritic cells are generated in the gut mucosa. Trends Immunol. 33, 42–48 (2012).
Spencer, S. P. et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science 343, 432–437 (2014).
Jaensson-Gyllenback, E. et al. Bile retinoids imprint intestinal CD103+ dendritic cells with the ability to generate gut-tropic T cells. Mucosal Immunol. 4, 438–447 (2011).
Wong, J. M., de Souza, R., Kendall, C. W., Emam, A. & Jenkins, D. J. Colonic health: fermentation and short chain fatty acids. J. Clin. Gastroenterol. 40, 235–243 (2006).
Meresse, B., Malamut, G. & Cerf-Bensussan, N. Celiac disease: an immunological jigsaw. Immunity 36, 907–919 (2012).
Knights, D., Lassen, K. G. & Xavier, R. J. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut 62, 1505–1510 (2013). This paper reports that correlating the genetic basis of Crohn's disease and immune responses to microbes may help to explain its anatomical localization.
Kaser, A., Zeissig, S. & Blumberg, R. S. Inflammatory bowel disease. Annu. Rev. Immunol. 28, 573–621 (2010).
Deuring, J. J. et al. Genomic ATG16L1 risk allele-restricted Paneth cell ER stress in quiescent Crohn's disease. Gut 63, 1081–1091 (2013).
Jostins, L. et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Raverdeau, M. & Mills, K. H. Modulation of T cell and innate immune responses by retinoic acid. J. Immunol. 192, 2953–2958 (2014).
Wehkamp, J. et al. Reduced Paneth cell α-defensins in ileal Crohn's disease. Proc. Natl Acad. Sci. USA 102, 18129–18134 (2005).
Wehkamp, J., Schmid, M. & Stange, E. F. Defensins and other antimicrobial peptides in inflammatory bowel disease. Curr. Opin. Gastroenterol. 23, 370–378 (2007).
Becker, C. et al. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J. Clin. Invest. 112, 693–706 (2003).
Maloy, K. J. & Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474, 298–306 (2011).
Chassaing, B. & Darfeuille-Michaud, A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology 140, 1720–1728 (2011).
Nguyen, H. T. et al. Crohn's disease-associated adherent invasive Escherichia coli modulate levels of microRNAs in intestinal epithelial cells to reduce autophagy. Gastroenterology 146, 508–519 (2014).
Wallace, K. L., Zheng, L. B., Kanazawa, Y. & Shih, D. Q. Immunopathology of inflammatory bowel disease. World J. Gastroenterol. 20, 6–21 (2014).
Parkes, M. The genetics universe of Crohn's disease and ulcerative colitis. Dig. Dis. 30 (Suppl. 1), 78–81 (2012). This paper compares the genetic basis of the human IBDs with discrete anatomical preferences.
Neurath, M. F., Finotto, S. & Glimcher, L. H. The role of Th1/Th2 polarization in mucosal immunity. Nature Med. 8, 567–573 (2002).
Biancheri, P. et al. Absence of a role for interleukin-13 in inflammatory bowel disease. Eur. J. Immunol. 44, 370–385 (2014).
Strober, W., Fuss, I. J. & Blumberg, R. S. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 20, 495–549 (2002).
Neurath, M. F. Animal models of inflammatory bowel diseases: illuminating the pathogenesis of colitis, ileitis and cancer. Dig. Dis. 30 (Suppl. 1), 91–94 (2012).
Rubtsov, Y. P. et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28, 546–558 (2008).
Saleh, M. & Elson, C. O. Experimental inflammatory bowel disease: insights into the host-microbiota dialog. Immunity 34, 293–302 (2011).
Fuss, I. J. et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J. Clin. Invest. 113, 1490–1497 (2004).
Heller, F., Fuss, I. J., Nieuwenhuis, E. E., Blumberg, R. S. & Strober, W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity 17, 629–638 (2002).
Kuhn, R., Lohler, J., Rennick, D., Rajewsky, K. & Muller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–279 (1993).
Glocker, E. O. et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N. Engl. J. Med. 361, 2033–2045 (2009).
Kontoyiannis, D., Pasparakis, M., Pizarro, T. T., Cominelli, F. & Kollias, G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 10, 387–398 (1999). This paper reports one of the few experimental models of IBD that affects the small intestine.
Matsumoto, S. et al. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut 43, 71–78 (1998).
Marini, M. et al. TNF-α neutralization ameliorates the severity of murine Crohn's-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc. Natl Acad. Sci. USA 100, 8366–8371 (2003).
McNamee, E. N. et al. Novel model of TH2-polarized chronic ileitis: the SAMP1 mouse. Inflamm. Bowel Dis. 16, 743–752 (2010).
Han, D. et al. Dendritic cell expression of the signaling molecule TRAF6 is critical for gut microbiota-dependent immune tolerance. Immunity 38, 1211–1222 (2013).
Travis, M. A. et al. Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449, 361–365 (2007).
Paidassi, H. et al. Preferential expression of integrin αvβ8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells. Gastroenterology 141, 1813–1820 (2011).
Palma, G. D. et al. Influence of milk-feeding type and genetic risk of developing coeliac disease on intestinal microbiota of infants: the PROFICEL study. PLoS ONE 7, e30791 (2012).
Sellitto, M. et al. Proof of concept of microbiome-metabolome analysis and delayed gluten exposure on celiac disease autoimmunity in genetically at-risk infants. PLoS ONE 7, e33387 (2012).
Ou, G. et al. Proximal small intestinal microbiota and identification of rod-shaped bacteria associated with childhood celiac disease. Am. J. Gastroenterol. 104, 3058–3067 (2009).
Sjoberg, V. et al. Intestinal T-cell responses in celiac disease — impact of celiac disease associated bacteria. PLoS ONE 8, e53414 (2013).
Bilimoria, K. Y. et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann. Surg. 249, 63–71 (2009).
American Cancer Society. Cancer Facts & Figures 2013. American Cancer Society [online] (2013).
O'Keefe, S. J. Nutrition and colonic health: the critical role of the microbiota. Curr. Opin. Gastroenterol. 24, 51–58 (2008).
Bongers, G. et al. Interplay of host microbiota, genetic perturbations, and inflammation promotes local development of intestinal neoplasms in mice. J. Exp. Med. 211, 457–472 (2014).
Huber, S. et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491, 259–263 (2012).
Martin, J. C. et al. Interleukin-22 binding protein (IL-22BP) is constitutively expressed by a subset of conventional dendritic cells and is strongly induced by retinoic acid. Mucosal Immunol. 7, 101–113 (2014).
Jang, M. H. et al. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc. Natl Acad. Sci. USA 101, 6110–6115 (2004).
Rescigno, M. et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nature Immunol. 2, 361–367 (2001).
Niess, J. H. et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, 254–258 (2005).
Chieppa, M., Rescigno, M., Huang, A. Y. & Germain, R. N. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J. Exp. Med. 203, 2841–2852 (2006).
Arques, J. L. et al. Salmonella induces flagellin- and MyD88-dependent migration of bacteria-capturing dendritic cells into the gut lumen. Gastroenterology 137, 579–587, 587 e1-2 (2009).
Farache, J. et al. Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity 38, 581–595 (2013).
McDole, J. R. et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483, 345–349 (2012). This is a state-of-the-art study using in vivo imaging which suggests how antigen may gain access via goblet cells to DCs in the small intestine but not in the colon.
Yoshida, M. et al. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity 20, 769–783 (2004).
Mazzini, E., Massimiliano, L., Penna, G. & Rescigno, M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1+ macrophages to CD103+ dendritic cells. Immunity 40, 248–261 (2014).
Arijs, I. et al. Mucosal gene expression of cell adhesion molecules, chemokines, and chemokine receptors in patients with inflammatory bowel disease before and after infliximab treatment. Am. J. Gastroenterol. 106, 748–761 (2011).
Papadakis, K. A. et al. CCR9-positive lymphocytes and thymus-expressed chemokine distinguish small bowel from colonic Crohn's disease. Gastroenterology 121, 246–254 (2001).
Johansson-Lindbom, B. et al. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J. Exp. Med. 198, 963–969 (2003).
Lindner, C. et al. Age, microbiota, and T cells shape diverse individual IgA repertoires in the intestine. J. Exp. Med. 209, 365–377 (2012).
Stenstad, H., Svensson, M., Cucak, H., Kotarsky, K. & Agace, W. W. Differential homing mechanisms regulate regionalized effector CD8αβ+ T cell accumulation within the small intestine. Proc. Natl Acad. Sci. USA 104, 10122–10127 (2007). This detailed study defines how CCL25 controls T cell homing to the small intestine but not to the colon, as well as describing differences within the small intestine itself.
Pan, J. et al. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J. Immunol. 165, 2943–2949 (2000).
Kunkel, E. J. et al. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J. Clin. Invest. 111, 1001–1010 (2003).
Cook, D. N. et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity 12, 495–503 (2000).
Iwasaki, A. & Kelsall, B. L. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3α, MIP-3β, and secondary lymphoid organ chemokine. J. Exp. Med. 191, 1381–1394 (2000).
Wang, C., Kang, S. G., Lee, J., Sun, Z. & Kim, C. H. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal Immunol. 2, 173–183 (2009).
Kitamura, K., Farber, J. M. & Kelsall, B. L. CCR6 marks regulatory T cells as a colon-tropic, IL-10-producing phenotype. J. Immunol. 185, 3295–3304 (2010).
Salazar-Gonzalez, R. M. et al. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer's patches. Immunity 24, 623–632 (2006).
McDonald, K. G. et al. CC chemokine receptor 6 expression by B lymphocytes is essential for the development of isolated lymphoid follicles. Am. J. Pathol. 170, 1229–1240 (2007).
Varona, R. et al. CCR6-deficient mice have impaired leukocyte homeostasis and altered contact hypersensitivity and delayed-type hypersensitivity responses. J. Clin. Invest. 107, R37–R45 (2001).
Zhao, X. et al. CCL9 is secreted by the follicle-associated epithelium and recruits dome region Peyer's patch CD11b+ dendritic cells. J. Immunol. 171, 2797–2803 (2003).
Cha, H. R. et al. Downregulation of Th17 cells in the small intestine by disruption of gut flora in the absence of retinoic acid. J. Immunol. 184, 6799–6806 (2010).
Chang, S. Y. et al. Lack of retinoic acid leads to increased langerin-expressing dendritic cells in gut-associated lymphoid tissues. Gastroenterology 138, 1468–1478, 1478 e1-6 (2010).
Coombes, J. L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Iwata, M. et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 (2004).
Klebanoff, C. A. et al. Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. J. Exp. Med. 210, 1961–1976 (2013).
Mora, J. R. et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314, 1157–1160 (2006).
Mora, J. R., Iwata, M. & von Andrian, U. H. Vitamin effects on the immune system: vitamins A and D take centre stage. Nature Rev. Immunol. 8, 685–698 (2008).
Mucida, D. et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317, 256–260 (2007).
Sun, C. M. et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204, 1775–1785 (2007).
Suzuki, K. et al. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity 33, 71–83 (2010).
Takahashi, H. et al. TGF-β and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nature Immunol. 13, 587–595 (2012).
Hammerschmidt, S. I. et al. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J. Exp. Med. 205, 2483–2490 (2008).
Molenaar, R. et al. Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells is controlled by dietary vitamin A. J. Immunol. 186, 1934–1942 (2011).
Kiss, E. A. & Diefenbach, A. Role of the aryl hydrocarbon receptor in controlling maintenance and functional programs of RORγt+ innate lymphoid cells and intraepithelial lymphocytes. Front. Immunol. 3, 124 (2012).
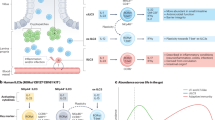
Veldhoen, M. & Brucklacher-Waldert, V. Dietary influences on intestinal immunity. Nature Rev. Immunol. 12, 696–708 (2012). This review discusses the effects of AHR ligands and other dietary components on the function of intestinal immune cells.
Lee, J. S. et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nature Immunol. 13, 144–151 (2012).
Qiu, J. et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36, 92–104 (2012).
Li, Y. et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147, 629–640 (2011).
MacDonald, T. T. & Carter, P. B. Requirement for a bacterial flora before mice generate cells capable of mediating the DTH reaction to sheep red blood cells. J. Immunol. 122, 2624–2629 (1979).
Talham, G. L., Jiang, H. Q., Bos, N. A. & Cebra, J. J. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect. Immun. 67, 1992–2000 (1999).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Goto, Y. et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity 40, 594–607 (2014).
Zelante, T. et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385 (2013).
Atarashi, K. et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236 (2013). This study describes the presence of bacteria in the human colon that drive the selective generation of T Reg cells.
Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011).
Round, J. L. & Mazmanian, S. K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl Acad. Sci. USA 107, 12204–12209 (2010).
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Singh, N. et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40, 128–139 (2014).
Cadwell, K. et al. Virus-plus-susceptibility gene interaction determines Crohn's disease gene Atg16L1 phenotypes in intestine. Cell 141, 1135–1145 (2010).
Virgin, H. W. The virome in mammalian physiology and disease. Cell 157, 142–150 (2014).
Ahmed, S. et al. Mucosa-associated bacterial diversity in relation to human terminal ileum and colonic biopsy samples. Appl. Environ. Microbiol. 73, 7435–7442 (2007).
Hu, S. et al. Regional differences in colonic mucosa-associated microbiota determine the physiological expression of host heat shock proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G1266–G1275 (2010). This study describes some of the immunological consequences of anatomical differences in the composition of the intestinal microbiota.
Belkaid, Y. & Hand, T. W. Role of the microbiota in immunity and inflammation. Cell 157, 121–141 (2014).
Unthank, J. L. & Bohlen, H. G. Lymphatic pathways and role of valves in lymph propulsion from small intestine. Am. J. Physiol. 254, G389–G398 (1988).
Miller, M. J., McDole, J. R. & Newberry, R. D. Microanatomy of the intestinal lymphatic system. Ann. NY Acad. Sci. 1207 (Suppl. 1), E21–E28 (2010).
Kawashima, Y., Sugimura, M., Hwang, Y.-C. & Kudo, N. The lymph system in mice. Japanese J. Veterinary Res. 12, 69–78 (1964).
Acknowledgements
The authors would like to thank A. Sasor and E. Toth at Skåne University Hospital, Sweden, for the intestinal section and endoscopy images, respectively. W.W.A. is supported by grants from the Swedish Medical Research Council, the Swedish National Health Service and a Sapere Aude senior researcher grant from the Danish Research Council. A.M.M is supported by grants from the Medical Research Council, UK, the Wellcome Trust and Tenovus Scotland.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Paneth cells
-
Specialized epithelial cells located just below the epithelial stem cells in the small intestinal crypts of Lieberkühn. They are a rich source of antimicrobial peptides that preserve crypt sterility and protect the epithelial stem cell niche.
- Goblet cells
-
Specialized epithelial cells that produce mucus.
- Submucosa
-
The layer of the intestine that is immediately below the mucosa and above the external muscle. Peyer's patches and colonic patches are located in the submucosa.
- Coeliac disease
-
An inflammatory disorder of the duodenum and jejunum that is caused by immune responses to specific peptides that are found within the α-gliadin component of wheat gluten. Pathology includes the loss of villus architecture with reduced area of the surface epithelium, which leads to malabsorption.
- Defensins
-
Small cationic proteins with antimicrobial properties that are produced by leukocytes and epithelial cells, such as Paneth cells.
- Crohn's disease
-
An inflammatory bowel disease that is characterized by chronic transmural inflammation and associated granuloma formation. Pathology can affect all parts of the digestive tract, in particular, the colon and terminal ileum.
- Follicle-associated epithelium
-
(FAE). A layer of columnar epithelial cells covering the surface of gut-associated lymphoid tissues such as Peyer's patches, the appendix and isolated lymphoid follicles. The FAE contains several immune cell populations and microfold cells.
- Microfold cells
-
(M cells). Specialized epithelial cells found within the follicle-associated epithelium that covers gut-associated lymphoid tissues. M cells lack microvilli and an overlying glycocalyx, and they are specialized in the uptake of bacteria and other particulate antigens, transporting them to neighbouring antigen- presenting cells. They represent a major site of entry for many viral and bacterial intestinal pathogens.
- Subepithelial dome
-
(SED). The area directly beneath the follicular- associated epithelium of gut-associated lymphoid tissues. It is rich in antigen-presenting cells and B cells.
- Caecal patches
-
Areas of organized lymphoid tissue found in the submucosa of the caecum in mice that are thought to be equivalent to the human appendix and to be responsible for generating colon-homing IgA+ plasma cells. The removal of caecal patches can prevent experimental inflammatory bowel disease.
- Colonic patches
-
Organized lymphoid tissues that are found in the submucosa of the colon. They contain both B cell and T cell areas, and develop before birth.
- Solitary isolated lymphoid tissues
-
(SILTs). The collective term for small lymphoid follicle aggregates that are found in the mucosa of the intestine. They include both cryptopatches and more mature isolated lymphoid follicles.
- Cryptopatches
-
Small organized areas of lymphoid tissue that are found in the wall of the intestine. Cryptopatches contain dendritic cells and lymphoid tissue inducer cells, and they are thought to be immature forms of isolated lymphoid follicles.
- Isolated lymphoid follicles
-
(ILFs). Mature lymphoid aggregates that are found in the mucosa. They primarily consist of B cells, dendritic cells and innate lymphoid cells, and they are covered by a follicle-associated epithelium. ILFs are thought to have a role in initiating local IgA responses and they are found throughout the intestine.
- Mesenteric lymph nodes
-
(MLNs). The series of lymph nodes draining the small intestine and upper colon. Human MLNs are found throughout the intestinal mesentery, whereas in the mouse, they consist of a string of four or five lymph nodes.
- Caudal lymph node
-
A lymph node found in the abdomen near the bifurcation of the aorta that is responsible for draining lymph from the descending colon and rectum.
- Innate lymphoid cells
-
(ILCs). A relatively recently identified population of non-T, non-B lymphocytes that are believed to have a central role in early innate immune responses in the intestinal mucosa. They have been subdivided into three main groups according to whether they express T helper 1 (TH1)-type, TH2-type or TH17-type transcription factors and cytokines.
- Segmented filamentous bacteria
-
(SFB). Commensal bacteria that are found as part of the normal microbiota in certain mouse facilities. Currently unculturable, these anaerobes colonize the terminal ileum of rodents, adhering to ileal enterocytes, and they have a major impact on the development and composition of immune cell populations in the small intestine.
- Mucosal-associated invariant T cells
-
(MAIT cells). Cells that express a semi-invariant T cell receptor (TCR) comprising the canonical TCR Vα7.2 and Jα33 in humans, and Vα19 and Jα33 in mice. They are predominantly found in the human jejunum and are believed to recognize vitamin B metabolites presented by MHC class I-related protein.
- Invariant natural killer T cell
-
(iNKT cell). T cells that express an invariant form of the αβ T cell receptor that recognizes glycolipid antigens presented by the CD1d molecule. They produce cytokines at an early stage in immune responses and may contribute to intestinal inflammation.
- Lymphotoxin-β receptor–Fc fusion protein
-
A fusion protein comprising the lymphotoxin-β receptor linked to the Fc portion of an immunoglobulin heavy chain. When given to pregnant mice, it prevents the development of Peyer's patches and other components of the gut-associated lymphoid tissue in the offspring by specifically blocking access of the α1β2 isoform of lymphotoxin to the LTβ receptor.
- Plasmacytoid DCs
-
(Plasmacytoid dendritic cells; pDCs). A population of dendritic cells with the appearance of plasma cells and a specialized ability to produce type I interferons in response to viruses and Toll-like receptor ligation, but little or no antigen presentation activity.
- Ulcerative colitis
-
One of the two major forms of human inflammatory bowel disease. It presents as a continuous area of inflammation that is restricted to the large intestine and is characterized by erythema, superficial ulceration and pseudopolyps.
- Dysbiosis
-
An imbalance in the composition of the microbial species that are normally found in the intestine. It is associated with alterations in immune function and susceptibility to inflammatory diseases, allergies and metabolic conditions.
- Dextran sodium sulphate
-
(DSS). Sodium salt of dextran that causes an acute colitis in rodents when administered orally.
Rights and permissions
About this article
Cite this article
Mowat, A., Agace, W. Regional specialization within the intestinal immune system. Nat Rev Immunol 14, 667–685 (2014). https://doi.org/10.1038/nri3738
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3738
This article is cited by
-
A body–brain circuit that regulates body inflammatory responses
Nature (2024)
-
Nurturing gut health: role of m6A RNA methylation in upholding the intestinal barrier
Cell Death Discovery (2024)
-
TLR7-dependent eosinophil degranulation links psoriatic skin inflammation to small intestinal inflammatory changes in mice
Experimental & Molecular Medicine (2024)
-
Malvidin alleviates LPS-induced septic intestinal injury through the nuclear factor erythroid 2-related factor 2/reactive oxygen species/NLRP3 inflammasome pathway
Inflammopharmacology (2024)
-
Immunomodulating effects of the single bacterial strain therapy EDP1815 on innate and adaptive immune challenge responses — a randomized, placebo-controlled clinical trial
Immunologic Research (2024)