Key Points
-
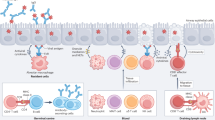
Influenza virus infection is detected by multiple pattern recognition receptors. Within the infected cells, retinoic acid-inducible gene I (RIG-I) detects the 5′-triphosphorylated RNA of replicating viral genomes, whereas in plasmacytoid dendritic cells and B cells Toll-like receptor 7 (TLR7) detects RNA that is associated with incoming virions. TLR3 is expressed in airway epithelial cells and macrophages and detects RNA that is associated with infected cells.
-
The activation of nucleic acid sensors leads to the expression of type I and type III interferons, which in turn stimulate the expression of hundreds of interferon-stimulated genes in neighbouring cells that induce antiviral state.
-
In myeloid cells, matrix 2 (M2) ion channel activity of influenza virus stimulates the NOD-, LRR- and pyrin domain-containing 3 (NLRP3) inflammasome, resulting in the activation of caspase 1 and the cleavage and release of interleukin-1β (IL-1β) and IL-18. Phagocytosis of influenza virus-infected cells containing PB1-F2 fibrils also triggers activation of the NLRP3 inflammasome. The NLRP3 inflammasome and IL-1β have an important role in host tolerance to influenza virus infection in response to high-dose viral challenge. IL-1β and IL-1R signalling promotes antiviral B cell and T cell responses after low-dose viral challenge.
-
Commensal bacteria provide signals that set the activation threshold for the induction of adaptive immune responses to influenza virus. Bacterial components drive the expression of the genes encoding pro-IL-1β and NLRP3, as well as interferon-stimulated genes, to promote the robust stimulation of B cell and T cell responses upon influenza virus infection.
-
Innate immune responses confer protection either by stimulating antiviral defence genes or by promoting disease tolerance of host tissues. Therapeutic approaches to combating influenza virus-initiated diseases should consider promoting both of these protective strategies.
Abstract
Influenza viruses are a major pathogen of both humans and animals. Recent studies using gene-knockout mice have led to an in-depth understanding of the innate sensors that detect influenza virus infection in a variety of cell types. Signalling downstream of these sensors induces distinct sets of effector mechanisms that block virus replication and promote viral clearance by inducing innate and adaptive immune responses. In this Review, we discuss the various ways in which the innate immune system uses pattern recognition receptors to detect and respond to influenza virus infection. We consider whether the outcome of innate sensor stimulation promotes antiviral resistance or disease tolerance, and propose rational treatment strategies for the acute respiratory disease that is caused by influenza virus infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Manicassamy, B. et al. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc. Natl Acad. Sci. 107, 11531–11536 (2010).
Perrone, L. A., Plowden, J. K., García-Sastre, A., Katz, J. M. & Tumpey, T. M. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 4, e1000115 (2008).
Hogner, K. et al. Macrophage-expressed IFN-β contributes to apoptotic alveolar epithelial cell injury in severe influenza virus pneumonia. PLoS Pathog. 9, e1003188 (2013).
Kallfass, C., Lienenklaus, S., Weiss, S. & Staeheli, P. Visualizing the beta interferon response in mice during infection with influenza A viruses expressing or lacking nonstructural protein 1. J. Virol. 87, 6925–6930 (2013).
Jewell, N. A. et al. Differential type I interferon induction by respiratory syncytial virus and influenza a virus in vivo. J. Virol. 81, 9790–9800 (2007).
Gazit, R. et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nature Immunol. 7, 517–523 (2006).
Hashimoto, Y., Moki, T., Takizawa, T., Shiratsuchi, A. & Nakanishi, Y. Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J. Immunol. 178, 2448–2457 (2007).
Braciale, T. J., Sun, J. & Kim, T. S. Regulating the adaptive immune response to respiratory virus infection. Nature Rev. Immunol. 12, 295–305 (2012).
Medzhitov, R., Schneider, D. S. & Soares, M. P. Disease tolerance as a defense strategy. Science 335, 936–941 (2012).
Raberg, L., Sim, D. & Read, A. F. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 318, 812–814 (2007).
Ayres, J. S. & Schneider, D. S. Tolerance of infections. Annu. Rev. Immunol. 30, 271–294 (2012).
Paiardini, M., Pandrea, I., Apetrei, C. & Silvestri, G. Lessons learned from the natural hosts of HIV-related viruses. Annu. Rev. Med. 60, 485–495 (2009).
Janeway, C. A. Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54, 1–13 (1989).
Medzhitov, R. Toll-like receptors and innate immunity. Nature Rev. Immunol. 1, 135–145 (2001).
Pang, I. K. & Iwasaki, A. Control of antiviral immunity by pattern recognition and the microbiome. Immunol. Rev. 245, 209–226 (2012).
Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001).
Pichlmair, A. et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314, 997–1001 (2006).
Wisskirchen, C., Ludersdorfer, T. H., Muller, D. A., Moritz, E. & Pavlovic, J. The cellular RNA helicase UAP56 is required for prevention of double-stranded RNA formation during influenza A virus infection. J. Virol. 85, 8646–8655 (2011).
Schulz, O. et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433, 887–892 (2005).
Le Goffic, R. et al. Cutting Edge: Influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J. Immunol. 178, 3368–3372 (2007).
Guillot, L. et al. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 280, 5571–5580 (2005).
Le Goffic, R. et al. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2, 0526 (2006). This study demonstrates a paradoxical role of TLR3 in promoting both antiviral resistance and inflammation, but despite an increase in viral load, Tlr3−/− mice had a survival advantage owing to reduced inflammation.
Heer, A. K. et al. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J. Immunol. 178, 2182–2191 (2007).
Lund, J. M. et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl Acad. Sci. USA 101, 5598–5603 (2004).
Diebold, S. S., Kaisho, T., Hemmi, H., Akira, S. & Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 (2004).
Honda, K. et al. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature 434, 1035–1040 (2005).
Sasai, M., Linehan, M. M. & Iwasaki, A. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science 329, 1530–1534 (2010).
Lande, R. et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449, 564–569 (2007).
Seo, S. U. et al. MyD88 signaling is indispensable for primary influenza A virus infection but dispensable for secondary infection. J. Virol. 84, 12713–12722 (2010).
Jeisy-Scott, V. et al. J. Virol. 86, 10988–10998 (2012).
Koyama, S. et al. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J. Immunol. 179, 4711–4720 (2007).
Ablasser, A. et al. Selection of molecular structure and delivery of RNA oligonucleotides to activate TLR7 versus TLR8 and to induce high amounts of IL-12p70 in primary human monocytes. J. Immunol. 182, 6824–6833 (2009).
Kato, H. et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23, 19–28 (2005).
Hornung, V. et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314, 994–997 (2006).
Rehwinkel, J. et al. RIG-I detects viral genomic RNA during negative-strand RNA virus infection. Cell 140, 397–408 (2010).
Baum, A., Sachidanandam, R. & Garcia-Sastre, A. Preference of RIG-I for short viral RNA molecules in infected cells revealed by next-generation sequencing. Proc. Natl Acad. Sci. USA 107, 16303–16308 (2010).
Luo, D. et al. Structural insights into RNA recognition by RIG-I. Cell 147, 409–422 (2011).
Jiang, F. et al. Structural basis of RNA recognition and activation by innate immune receptor RIG-I. Nature 479, 423–427 (2011).
Kowalinski, E. et al. Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell 147, 423–435 (2011).
Onomoto, K. et al. Critical role of an antiviral stress granule containing RIG-I and PKR in viral detection and innate immunity. PLoS ONE 7, e43031 (2012). This study demonstrates that the stress granules that form in infected cells serve as a hub for viral RNA accumulation and RIG-I signalling.
Pang, I. K., Ichinohe, T. & Iwasaki, A. IL-1R signaling in dendritic cells replaces pattern-recognition receptors in promoting CD8+ T cell responses to influenza A virus. 14, 246–253 (2013).
Gack, M. U. et al. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 5, 439–449 (2009).
Bergsbaken, T., Fink, S. L. & Cookson, B. T. Pyroptosis: host cell death and inflammation. Nature Rev. Microbiol. 7, 99–109 (2009).
Martinon, F., Mayor, A. & Tschopp, J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265 (2009).
Guarda, G. et al. Differential expression of NLRP3 among hematopoietic cells. J. Immunol. 186, 2529–2534 (2011).
Pothlichet, J. et al. Type I IFN triggers RIG-I/TLR3/NLRP3-dependent inflammasome activation in influenza A virus infected cells. PLoS Pathog. 9, e1003256 (2013).
Thomas, P. G. et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30, 566–575 (2009).
Ichinohe, T., Pang, I. K. & Iwasaki, A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nature Immunol. 11, 404–410 (2010).
McAuley, J. L. et al. Activation of the NLRP3 inflammasome by IAV virulence protein PB1-F2 contributes to severe pathophysiology and disease. PLoS Pathog. 9, e1003392 (2013).
Ichinohe, T., Yamazaki, T., Koshiba, T. & Yanagi, Y. Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proc. Natl Acad. Sci. USA 110, 17963–17968 (2013).
Tal, M. C. & Iwasaki, A. Mitoxosome: a mitochondrial platform for cross-talk between cellular stress and antiviral signaling. Immunol. Rev. 243, 215–234 (2011).
Allen, I. C. et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30, 556–565 (2009).
Van Der Sluijs, K. F. et al. Enhanced viral clearance in interleukin-18 gene-deficient mice after pulmonary infection with influenza A virus. Immunology 114, 112–120 (2005).
Schmitz, N., Kurrer, M., Bachmann, M. F. & Kopf, M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J. Virol. 79, 6441–6448 (2005).
Ichinohe, T., Lee, H. K., Ogura, Y., Flavell, R. & Iwasaki, A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 206, 79–87 (2009).
Staeheli, P., Haller, O., Boll, W., Lindenmann, J. & Weissmann, C. Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to influenza virus. Cell 44, 147–158 (1986).
Zurcher, T., Pavlovic, J. & Staeheli, P. Mouse Mx2 protein inhibits vesicular stomatitis virus but not influenza virus. Virology 187, 796–800 (1992).
Hefti, H. P. et al. Human MxA protein protects mice lacking a functional alpha/beta interferon system against La Crosse virus and other lethal viral infections. J. Virol. 73, 6984–6991 (1999).
Turan, K. et al. Nuclear MxA proteins form a complex with influenza virus NP and inhibit the transcription of the engineered influenza virus genome. Nucleic Acids Res. 32, 643–652 (2004).
Pavlovic, J. et al. Enhanced virus resistance of transgenic mice expressing the human MxA protein. J. Virol. 69, 4506–4510 (1995).
Goujon, C. et al. Human MX2 is an interferon-induced post-entry inhibitor of HIV-1 infection. Nature 502, 559–562 (2013).
Liu, Z. et al. The interferon-inducible MxB protein inhibits HIV-1 infection. Cell Host Microbe 14, 398–410 (2013).
Brass, A. L. et al. The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell 139, 1243–1254 (2009). This study uses a functional genomic screen and shows that IFITM1, IFITM2 and IFITM3 restrict an early step in the replication of influenza A virus.
Bailey, C. C., Huang, I. C., Kam, C. & Farzan, M. Ifitm3 limits the severity of acute influenza in mice. PLoS Pathog. 8, e1002909 (2012).
Everitt, A. R. et al. IFITM3 restricts the morbidity and mortality associated with influenza. Nature 484, 519–523 (2012). References 64 and 65 show that despite the presence of other IFITM proteins, Ifitm3−/− mice succumb to influenza virus infection owing to impaired viral control and severe pathology.
Sadler, A. J. & Williams, B. R. Interferon-inducible antiviral effectors. Nature Rev. Immunol. 8, 559–568 (2008).
Silverman, R. H. Viral encounters with 2′,5′-oligoadenylate synthetase and RNase L during the interferon antiviral response. J. Virol. 81, 12720–12729 (2007).
Kumar, A., Haque, J., Lacoste, J., Hiscott, J. & Williams, B. R. Double-stranded RNA-dependent protein kinase activates transcription factor NF-κB by phosphorylating IκB. Proc. Natl Acad. Sci. USA 91, 6288–6292 (1994).
Schulz, O. et al. Protein kinase R contributes to immunity against specific viruses by regulating interferon mRNA integrity. Cell Host Microbe 7, 354–361 (2010).
Balachandran, S. et al. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13, 129–141 (2000).
Dauber, B. et al. Influenza B virus ribonucleoprotein is a potent activator of the antiviral kinase PKR. PLoS Pathog. 5, e1000473 (2009).
Schoggins, J. W. et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 505, 691–695 (2013). This study uses an ectopic expression assay to screen a library of more than 350 human ISGs for their effects on 14 viruses representing seven families and 11 genera. It reveals several new ISGs that are capable of restricting influenza virus replication. It is also a great resource paper.
Wang, X., Hinson, E. R. & Cresswell, P. The interferon-inducible protein viperin inhibits influenza virus release by perturbing lipid rafts. Cell Host Microbe 2, 96–105 (2007).
Blanc, M. et al. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity 38, 106–118 (2013).
Liu, S. Y. et al. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity 38, 92–105 (2013).
Di Pietro, A. et al. TRIM22 inhibits influenza A virus infection by targeting the viral nucleoprotein for degradation. J. Virol. 87, 4523–4533 (2013).
Durfee, L. A., Lyon, N., Seo, K. & Huibregtse, J. M. The ISG15 conjugation system broadly targets newly synthesized proteins: implications for the antiviral function of ISG15. Mol. Cell 38, 722–732 (2010).
Lenschow, D. J. et al. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc. Natl Acad. Sci. USA 104, 1371–1376 (2007).
Kohlmeier, J. E., Cookenham, T., Roberts, A. D., Miller, S. C. & Woodland, D. L. Type I interferons regulate cytolytic activity of memory CD8+ T cells in the lung airways during respiratory virus challenge. Immunity 33, 96–105 (2010).
Der, S. D., Zhou, A., Williams, B. R. & Silverman, R. H. Identification of genes differentially regulated by interferon α, β, or γ using oligonucleotide arrays. Proc. Natl Acad. Sci. USA 95, 15623–15628 (1998).
Mordstein, M. et al. Interferon-λ contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog. 4, e1000151 (2008).
Durbin, J. E. et al. Type I IFN modulates innate and specific antiviral immunity. J. Immunol. 164, 4220–4228 (2000).
Hermesh, T., Moran, T. M., Jain, D. & Lopez, C. B. Granulocyte colony-stimulating factor protects mice during respiratory virus infections. PLoS ONE 7, e37334 (2012).
Lauder, S. N. et al. Interleukin-6 limits influenza-induced inflammation and protects against fatal lung pathology. Eur. J. Immunol. 43, 2613–2625 (2013).
Denton, A. E., Doherty, P. C., Turner, S. J. & La Gruta, N. L. IL-18, but not IL-12, is required for optimal cytokine production by influenza virus-specific CD8+ T cells. Eur. J. Immunol. 37, 368–375 (2007).
Sun, K., Torres, L. & Metzger, D. W. A detrimental effect of interleukin-10 on protective pulmonary humoral immunity during primary influenza A virus infection. J. Virol. 84, 5007–5014 (2010).
McKinstry, K. K. et al. IL-10 deficiency unleashes an influenza-specific TH17 response and enhances survival against high-dose challenge. J. Immunol. 182, 7353–7363 (2009).
Sun, J., Madan, R., Karp, C. L. & Braciale, T. J. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nature Med. 15, 277–284 (2009).
Monticelli, L. A. et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nature Immunol. 12, 1045–1054 (2011).
Chang, Y. J. et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nature Immunol. 12, 631–638 (2011).
Carlson, C. M. et al. Transforming growth factor-β: activation by neuraminidase and role in highly pathogenic H5N1 influenza pathogenesis. PLoS Pathog. 6, e1001136 (2010).
Williams, A. E. et al. TGF-β prevents eosinophilic lung disease but impairs pathogen clearance. Microbes Infect. 7, 365–374 (2005).
Snelgrove, R. J. et al. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nature Immunol. 9, 1074–1083 (2008).
Crowe, C. R. et al. Critical role of IL-17RA in immunopathology of influenza infection. J. Immunol. 183, 5301–5310 (2009).
Szretter, K. J. et al. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J. Virol. 81, 2736–2744 (2007).
Blander, J. M. & Sander, L. E. Beyond pattern recognition: five immune checkpoints for scaling the microbial threat. Nature Rev. Immunol. 12, 215–225 (2012).
Nish, S. & Medzhitov, R. Host defense pathways: role of redundancy and compensation in infectious disease phenotypes. Immunity 34, 629–636 (2011).
Pang, I. K., Pillai, P. S. & Iwasaki, A. Efficient influenza A virus replication in the respiratory tract requires signals from TLR7 and RIG-I. Proc. Natl Acad. Sci. USA 110, 13910–13915 (2013).
Negishi, H. et al. Cross-interference of RLR and TLR signaling pathways modulates antibacterial T cell responses. Nature Immunol. 13, 659–666 (2012).
Edwards, A. D. et al. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8α+ DC correlates with unresponsiveness to imidazoquinolines. Eur. J. Immunol. 33, 827–833 (2003).
Geeraedts, F. et al. Superior immunogenicity of inactivated whole virus H5N1 influenza vaccine is primarily controlled by Toll-like receptor signalling. PLoS Pathog. 4, e1000138 (2008). This study provides definitive evidence for the role of TLR7 in generating protective antibody responses to whole killed influenza vaccine, and demonstrates that split vaccines are inferior owing to the loss of viral RNA during vaccine preparation.
Clingan, J. M. & Matloubian, M. B. Cell-intrinsic TLR7 signaling is required for optimal B cell responses during chronic viral infection. J. Immunol. 191, 810–818 (2013).
Kash, J. C. et al. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature 443, 578–581 (2006).
Kobasa, D. et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 445, 319–323 (2007).
Cilloniz, C. et al. Lethal influenza virus infection in macaques is associated with early dysregulation of inflammatory related genes. PLoS Pathog. 5, e1000604 (2009).
Baskin, C. R. et al. Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus. Proc. Natl Acad. Sci. USA 106, 3455–3460 (2009).
Palese, P. Influenza: old and new threats. Nature Med. 10, S82–87 (2004).
Imai, Y. et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133, 235–249 (2008).
Morita, M. et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell 153, 112–125 (2013). This study uses a mediator lipidomics and bioactive lipid screen and demonstrates the regulation of lipid metabolites that are generated during influenza virus infection. The study also identifies protectin D1 as a candidate antiviral molecule that can reduce viral load in vivo.
Tisoncik, J. R. et al. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 76, 16–32 (2012).
Calvaruso, V., Mazza, M. & Almasio, P. L. Pegylated-interferon-α2a in clinical practice: how to manage patients suffering from side effects. Expert Opin. Drug Saf 10, 429–435 (2011).
Hussell, T., Pennycook, A. & Openshaw, P. J. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol. 31, 2566–2573 (2001).
Brandes, M., Klauschen, F., Kuchen, S. & Germain, R. N. A systems analysis identifies a feedforward inflammatory circuit leading to lethal influenza infection. Cell 154, 197–212 (2013).
Cyster, J. G. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23, 127–159 (2005).
Teijaro, J. R. et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 146, 980–991 (2011).
Shirey, K. A. et al. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature 497, 498–502 (2013).
Shi, X. et al. PEGylated human catalase elicits potent therapeutic effects on H1N1 influenza-induced pneumonia in mice. Appl. Microbiol. Biotechnol. 97, 10025–10033 (2013).
Vlahos, R. et al. Inhibition of Nox2 oxidase activity ameliorates influenza A virus-induced lung inflammation. PLoS Pathog. 7, e1001271 (2011).
Thompson, W.W. et al. Influenza-associated hospitalizations in the United States. JAMA 292, 1333–1340 (2004).
Beadling, C. & Slifka, M. K. How do viral infections predispose patients to bacterial infections? Curr. Opin. Infecti. Diseases 17, 185–191 (2004).
McCullers, J. A. Insights into the interaction between influenza virus and pneumococcus. Clin. Microbiol. Rev. 19, 571–582 (2006).
Glezen, W. P., Greenberg, S. B., Atmar, R. L., Piedra, P. A. & Couch, R. B. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA 283, 499–505 (2000).
Horimoto, T. & Kawaoka, Y. Pandemic threat posed by avian influenza A viruses. Clin. Microbiol. Rev. 14, 129–149 (2001).
Tscherne, D. M. & Garcia-Sastre, A. Virulence determinants of pandemic influenza viruses. J. Clin. Invest. 121, 6–13 (2011).
Ungchusak, K. et al. Probable person-to-person transmission of avian influenza A (H5N1). New Engl. J. Med. 352, 333–340 (2005).
World Health Organization Global Influenza Program Surveillance Network. Evolution of H5N1 avian influenza viruses in Asia. Emerg. Infect. Diseases 11, 1515–1521 (2005).
Herfst, S. et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336, 1534–1541 (2012).
Imai, M. et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486, 420–428 (2012).
Staeheli, P., Grob, R., Meier, E., Sutcliffe, J. G. & Haller, O. Influenza virus-susceptible mice carry Mx genes with a large deletion or a nonsense mutation. Mol. Cell. Biol. 8, 4518–4523 (1988).
Koerner, I., Kochs, G., Kalinke, U., Weiss, S. & Staeheli, P. Protective role of beta interferon in host defense against influenza A virus. J. Virol. 81, 2025–2030 (2007).
Kaminski, M. M., Ohnemus, A., Cornitescu, M. & Staeheli, P. Plasmacytoid dendritic cells and Toll-like receptor 7-dependent signalling promote efficient protection of mice against highly virulent influenza A virus. J. General Virol. 93, 555–559 (2012). The authors demonstrate a key role of TLR7 and pDCs in antiviral defence, which has not previously been possible to study in inbred mice that lack MX1. This study indicates the importance of studying immune defence in the context of MX1-sufficient mice.
GeurtsvanKessel, C. H. et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b− but not plasmacytoid dendritic cells. J. Exp. Med. 205, 1621–1634 (2008).
Wolf, A. I. et al. Plasmacytoid dendritic cells are dispensable during primary influenza virus infection. J. Immunol. 182, 871–879 (2009).
Ichinohe, T. et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl Acad. Sci. USA 108, 5354–5359 (2011).
Shaw, M. H., Kamada, N., Kim, Y. G. & Nunez, G. Microbiota-induced IL-1β, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J. Exp. Med. 209, 251–258 (2012).
Hoshi, N. et al. MyD88 signalling in colonic mononuclear phagocytes drives colitis in IL-10-deficient mice. Nature Commun. 3, 1120 (2012).
Helft, J. et al. Cross-presenting CD103+ dendritic cells are protected from influenza virus infection. J. Clin. Invest. 122, 4037–4047 (2012).
Langlois, R. A., Varble, A., Chua, M. A., Garcia-Sastre, A. & tenOever, B. R. Hematopoietic-specific targeting of influenza A virus reveals replication requirements for induction of antiviral immune responses. Proc. Natl Acad. Sci. USA 109, 12117–12122 (2012).
Abt, M. C. et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 37, 158–170 (2012).
Zurcher, T., Pavlovic, J. & Staeheli, P. Nuclear localization of mouse Mx1 protein is necessary for inhibition of influenza virus. J. Virol. 66, 5059–5066 (1992).
Cilloniz, C. et al. Molecular signatures associated with Mx1-mediated resistance to highly pathogenic influenza virus infection: mechanisms of survival. J. Virol. 86, 2437–2446 (2012).
Garber, E. A., Hreniuk, D. L., Scheidel, L. M. & van der Ploeg, L. H. Mutations in murine Mx1: effects on localization and antiviral activity. Virology 194, 715–723 (1993).
Sadler, A. J. & Williams, B. R. Structure and function of the protein kinase R. Curr. Top. Microbiol. Immunol. 316, 253–292 (2007).
Jia, R. et al. Identification of an endocytic signal essential for the antiviral action of IFITM3. Cell. Microbiol. http://dx.doi.org/10.1111/cmi.12262 (2014).
Hinson, E. R. & Cresswell, P. The N-terminal amphipathic α-helix of viperin mediates localization to the cytosolic face of the endoplasmic reticulum and inhibits protein secretion. J. Biol. Chem. 284, 4705–4712 (2009).
Hinson, E. R. & Cresswell, P. The antiviral protein, viperin, localizes to lipid droplets via its N-terminal amphipathic α-helix. Proc. Natl Acad. Sci. USA 106, 20452–20457 (2009).
Hidaka, F. et al. A missense mutation of the Toll-like receptor 3 gene in a patient with influenza-associated encephalopathy. Clin. Immunol. 119, 188–194 (2006).
Esposito, S. et al. Toll-like receptor 3 gene polymorphisms and severity of pandemic A/H1N1/2009 influenza in otherwise healthy children. Virol. J. 9, 270 (2012).
Goodman, A. G. et al. The alpha/beta interferon receptor provides protection against influenza virus replication but is dispensable for inflammatory response signaling. J. Virol. 84, 2027–2037 (2010).
Price, G. E., Gaszewska-Mastarlarz, A. & Moskophidis, D. The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice. J. Virol. 74, 3996–4003 (2000).
Garcia-Sastre, A. et al. The role of interferon in influenza virus tissue tropism. J. Virol. 72, 8550–8558 (1998).
Woods, A. et al. Influenza virus-induced type I interferon leads to polyclonal B-cell activation but does not break down B-cell tolerance. J. Virol. 81, 12525–12534 (2007).
Acknowledgements
The authors would like to thank R. Medzhitov for critical discussion and the US National Institutes of Health (NIH) for its support of research in the laboratory (grant numbers AI081884, AI054359, AI062428 and AI064705). Research on influenza virus in the laboratory was supported in part by the National Institute of Allergy and Infectious Diseases, NIH, USA, under award number U54AI057160 to the Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research (MRCE), USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Pattern recognition receptors
-
(PRRs). Germ-line encoded host receptors that are membrane bound (such as Toll-like receptors) or soluble and present in the cytoplasm (such as RIG-I, MDA5 and NLRs) and sense pathogen-associated molecular patterns and initiate signalling cascades that lead to innate immune responses.
- Type I interferons
-
(Type I IFNs). Cytokines that are rapidly induced by virus infection, as well as by some bacterial infections. They act on the IFN receptor to limit viral replication and to enhance antigen-specific immune responses.
- Antigenic drift
-
A process by which circulating influenza virus genomes are constantly changing, which allows the virus to cause annual epidemics. Antigenic drift occurs when mutations accumulate in the haemagglutinin and neuraminidase genes that alter the antigenicity of these proteins such that the 'drifted' strains are no longer neutralized by antibodies that were specific for previously circulating strains.
- Antigenic shift
-
A process by which a new influenza A virus haemagglutinin subtype (with or without an accompanying new neuraminidase subtype) is introduced into the human population, which often lacks prior experience of and immunity to the subtype. Antigenic shift can occur as a result of the direct introduction of an influenza virus from an animal or avian host into humans or by the exchange or reassortment of gene segments between human and non-human influenza viruses when they co-infect animals or humans.
- Eicosanoids
-
Fatty acid derivatives, mainly derived from arachidonic acid precursors, that have a wide variety of biological activities. There are four main classes of eicosanoid — the prostaglandins, prostacyclins, thromboxanes and leukotrienes — that are derived from the activities of cyclooxygenases and lipoxygenases on membrane-associated fatty acid precursors.
- IFN-stimulated genes
-
(ISGs). These genes contain interferon (IFN)-responsive promoters and are responsible for the antiviral, antiproliferative and immunomodulatory properties of IFN. More than 400 such genes have been identified by microarray analysis. Some — such as protein kinase R, ribonuclease L, IFN-induced GTP-binding protein MX1 and ISG15 — have well-documented antiviral activities, but the precise biological function of the majority of these genes is unknown.
- Antiviral resistance
-
A host strategy to reduce viral burden by recognizing and eliminating viruses and virus-infected cells.
- Disease tolerance
-
A host strategy to reduce tissue damage inflicted by pathogens or the immune response to pathogens.
- Antiviral stress granules
-
Dense cytoplasmic aggregates of RNA and proteins that appear when a cell is under stress. Stress granules contain stalled translation initiation complexes and processing bodies, and are a site for the storage of transiently repressed mRNA.
- Inflammasome
-
A molecular complex of several proteins that upon assembly activates caspase 1, which in turn cleaves substrates including pro-interleukin-1 (pro-IL-1), thereby producing active IL-1.
- Pyroptosis
-
A form of programmed cell death that requires caspase 1 downstream of inflammasome activation. Pyroptosis is accompanied by pore formation and cell swelling, and is an inflammatory form of death.
- Mitofusin 2
-
A mitochondrial GTPase that is embedded in the outer membrane that participates in mitochondrial fusion.
- Innate lymphoid cells
-
(ILCs). A group of innate immune cells that are lymphoid in morphology and developmental origin, but lack properties of adaptive B cells and T cells such as recombined antigen-specific receptors. They function in the regulation of immunity, tissue homeostasis and inflammation in response to cytokine stimulation.
- Amphiregulin
-
An epidermal growth factor (EGF)-like growth factor that stimulates tissue remodelling and the growth of epithelial cells through binding to its receptor, EGFR. Amphiregulin has been shown to restore lung function after influenza virus infection.
- Airway hyperresponsiveness
-
A hyperreactivity of the airways that is initiated by exposure to a defined stimulus and is usually tolerated by normal individuals but that causes bronchoconstriction and inflammatory cell infiltration in allergic individuals.
- γδ T cells
-
T cells that express a T cell receptor that consists of a γ-chain and a δ-chain and that are involved in innate immune responses. γδ T cells are present in the skin, digestive tract and reproductive mucosa.
- Viability-associated PAMPs
-
(Vita-PAMPs). Members of a special class of pathogen-associated molecular patterns (PAMPs) that are recognized by the innate immune system to signify microbial life. These patterns differentiate dead and living microorganisms to allow for the scaling of appropriate immune responses based on the level of threat that the microbial signals represents.
- Cross-priming
-
The initiation of a CD8+ T cell response to an antigen that has been taken up by antigen-presenting cells and re-routed to the MHC class I presentation pathway.
- NADPH oxidase
-
An enzyme system that consists of multiple cytosolic and membrane-bound subunits. The complex is assembled in activated neutrophils mainly on the phagolysosomal membrane. NADPH oxidase uses electrons from NADPH to reduce molecular oxygen to form superoxide anions. Superoxide anions are enzymatically converted into hydrogen peroxide, which is converted by myeloperoxidase to hypochloric acid, a highly toxic and microbicidal agent.
Rights and permissions
About this article
Cite this article
Iwasaki, A., Pillai, P. Innate immunity to influenza virus infection. Nat Rev Immunol 14, 315–328 (2014). https://doi.org/10.1038/nri3665
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3665
This article is cited by
-
Cullin5 drives experimental asthma exacerbations by modulating alveolar macrophage antiviral immunity
Nature Communications (2024)
-
TRIM38 Induced in Respiratory Syncytial Virus-infected Cells Downregulates Type I Interferon Expression by Competing with TRIM25 to Bind RIG-I
Inflammation (2024)
-
Double-edged sword of JAK/STAT signaling pathway in viral infections: novel insights into virotherapy
Cell Communication and Signaling (2023)
-
TRAF3 activates STING-mediated suppression of EV-A71 and target of viral evasion
Signal Transduction and Targeted Therapy (2023)
-
A quantitative systems pharmacology model of the pathophysiology and treatment of COVID-19 predicts optimal timing of pharmacological interventions
npj Systems Biology and Applications (2023)