Key Points
-
The acute and chronic forms of graft-versus-host disease (GVHD) have limited the success of allogeneic haematopoietic stem cell transplantation (HSCT), resulting in high rates of mortality and morbidity.
-
The main focus of treatment approaches for GVHD include: direct targeting of alloreactive T cells and their subsets; inhibition of their function using cytokine-specific antibodies; inhibition of their signalling pathways; and interference with their recruitment and homing to target tissues.
-
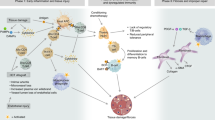
The activation of cellular and humoral components of the innate immune system owing to toxicity of the conditioning regimen or in response to certain gut microorganisms can augment acute GVHD. Strategies to decrease the toxicity of the conditioning regimen, manipulate the gut microbiome, control the release of damage-associated and pathogen-associated molecular patterns or inhibit the activity of their immune cell targets may be useful to prevent or treat GVHD.
-
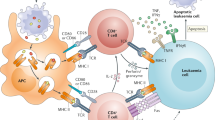
Recent studies have revisited the role of recipient versus donor and haematopoietic versus non-haematopoietic antigen-presenting cells (APCs) in the initiation of GVHD. This work suggests that several cell types may be responsible for alloantigen presentation in GVHD initiation. Therefore, strategies to impair antigen-presentation pathways in all APCs rather than deplete a single cell type may prove to be more effective in eliminating GVHD.
-
Our improved understanding of the role of B cells in acute and particularly chronic GVHD — in conjunction with improved tools for targeting B cells (such as those directed towards B cell receptors or their signalling pathways, B cell cytokine responsiveness and B cell–T cell cooperativity) — will allow the development of novel approaches for future clinical intervention in GVHD.
-
The inhibition of co-stimulatory molecules and their receptors remains a logical approach for the treatment or prevention of GVHD. The role of T cell co-inhibitory pathways, such as the programmed cell death protein 1 (PD1)–PD1 ligand 1 (PDL1) pathway, in GVHD is currently being investigated.
-
Immune regulatory cells — such as natural or induced regulatory T cells, tolerogenic dendritic cells, natural killer (NK) cells, NKT cells and myeloid-derived suppressor cells — can suppress GVHD in preclinical models. Several therapeutic approaches that exploit regulatory cells have already shown efficacy in early-stage clinical trials. Such strategies include: the in vivo infusion of regulatory T cells; the administration of low-dose interleukin-2 or histone deacetylase inhibitors to support the expansion of regulatory T cell populations in vivo; and the use of conditioning regimens that provide a relative increase in regulatory populations.
-
The future basic research and clinical trials in GVHD should specifically focus on approaches that inhibit GVHD while sparing (or even augmenting) graft-versus-tumour effects.
Abstract
Allogeneic haematopoietic stem cell transplantation is used to treat a variety of disorders, but its efficacy is limited by the occurrence of graft-versus-host disease (GVHD). The past decade has brought impressive advances in our understanding of the role of stimulatory and suppressive elements of the adaptive and innate immune systems from both the donor and the host in GVHD pathogenesis. New insights from basic immunology, preclinical models and clinical studies have led to novel approaches for prevention and treatment. This Review highlights the recent advances in understanding the pathophysiology of GVHD and its treatment, with a focus on manipulations of the immune system that are amenable to clinical application.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pasquini, M. C., Wang, Z., Horowitz, M. M. & Gale, R. P. 2010 report from the Center for International Blood and Marrow Transplant Research (CIBMTR): current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin. Transpl. 2010, 87–105 (2010).
Alyea, E. P. et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol. Blood Marrow Transplant. 12, 1047–1055 (2006).
Scott, B. L. et al. Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia 20, 128–135 (2006).
Barnes, D. W., Loutit, J. F. & Micklem, H. S. “Secondary disease” of radiation chimeras: a syndrome due to lymphoid aplasia. Ann. NY Acad. Sci. 99, 374–385 (1962).
Billingham, R. E. The biology of graft-versus-host reactions. Harvey Lect. 62, 21–78 (1966).
Broady, R. et al. Cutaneous GVHD is associated with the expansion of tissue-localized Th1 and not Th17 cells. Blood 116, 5748–5751 (2010).
Imanguli, M. M. et al. Increased T-bet+ cytotoxic effectors and type I interferon-mediated processes in chronic graft-versus-host disease of the oral mucosa. Blood 113, 3620–3630 (2009).
Murphy, W. J. et al. Differential effects of the absence of interferon-γ and IL-4 in acute graft-versus-host disease after allogeneic bone marrow transplantation in mice. J. Clin. Invest. 102, 1742–1748 (1998).
Nikolic, B., Lee, S., Bronson, R. T., Grusby, M. J. & Sykes, M. Th1 and Th2 mediate acute graft-versus-host disease, each with distinct end-organ targets. J. Clin. Invest. 105, 1289–1298 (2000). This article demonstrates that, despite the common belief, T H 2 cells are also important in the induction of acute GVHD.
Ratajczak, P. et al. Th17/Treg ratio in human graft-versus-host disease. Blood 116, 1165–1171 (2010).
Welniak, L. A., Blazar, B. R. & Murphy, W. J. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu. Rev. Immunol. 25, 139–170 (2007).
Ferrara, J. L., Levine, J. E., Reddy, P. & Holler, E. Graft-versus-host disease. Lancet 373, 1550–1561 (2009).
Sakoda, Y. et al. Donor-derived thymic-dependent T cells cause chronic graft-versus-host disease. Blood 109, 1756–1764 (2007).
Srinivasan, M. et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood 119, 1570–1580 (2012).
Hill, G. R. & Ferrara, J. L. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood 95, 2754–2759 (2000).
Imado, T. et al. The protective role of host Toll-like receptor-4 in acute graft-versus-host disease. Transplantation 90, 1063–1070 (2010).
Calcaterra, C. et al. Critical role of TLR9 in acute graft-versus-host disease. J. Immunol. 181, 6132–6139 (2008).
Heimesaat, M. M. et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut 59, 1079–1087 (2010).
Hossain, M. S. et al. Flagellin, a TLR5 agonist, reduces graft-versus-host disease in allogeneic hematopoietic stem cell transplantation recipients while enhancing antiviral immunity. J. Immunol. 187, 5130–5140 (2011).
Penack, O., Holler, E. & van den Brink, M. R. Graft-versus-host disease: regulation by microbe-associated molecules and innate immune receptors. Blood 115, 1865–1872 (2010).
Chakraverty, R. et al. An inflammatory checkpoint regulates recruitment of graft-versus-host reactive T cells to peripheral tissues. J. Exp. Med. 203, 2021–2031 (2006).
Heimesaat, M. M. et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J. Immunol. 177, 8785–8795 (2006).
Lupp, C. et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2, 119–129 (2007).
Gerbitz, A. et al. Probiotic effects on experimental graft-versus-host disease: let them eat yogurt. Blood 103, 4365–4367 (2004).
Loiarro, M. et al. Pivotal advance: inhibition of MyD88 dimerization and recruitment of IRAK1 and IRAK4 by a novel peptidomimetic compound. J. Leukoc. Biol. 82, 801–810 (2007).
Wilhelm, K. et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nature Med. 16, 1434–1438 (2010).
Lee, K. H. et al. P2X7 receptor polymorphism and clinical outcomes in HLA-matched sibling allogeneic hematopoietic stem cell transplantation. Haematologica 92, 651–657 (2007).
Li, H. et al. Graft-versus-host disease is independent of innate signaling pathways triggered by pathogens in host hematopoietic cells. J. Immunol. 186, 230–241 (2011).
Matte, C. C. et al. Donor APCs are required for maximal GVHD but not for GVL. Nature Med. 10, 987–992 (2004).
Shlomchik, W. D. et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science 285, 412–415 (1999). A benchmark study demonstrating the role of host APCs in GVHD and the potential clinical applications of this finding.
Anderson, B. E. et al. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood 105, 2227–2234 (2005).
Duffner, U. A. et al. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J. Immunol. 172, 7393–7398 (2004).
Markey, K. A. et al. Conventional dendritic cells are the critical donor APC presenting alloantigen after experimental bone marrow transplantation. Blood 113, 5644–5649 (2009).
Teshima, T. et al. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nature Med. 8, 575–581 (2002).
Koyama, M. et al. Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versus-host disease. Nature Med. 18, 135–142 (2012).
Toubai, T. et al. Induction of acute GVHD by sex-mismatched H-Y antigens in the absence of functional radio-sensitive host hematopoietic-derived antigen presenting cells. Blood 18 Nov 2011 (doi:10.1182/blood-2011-10-384057). References 35 and 36 demonstrate the role of non-haematopoietic APCs in the induction of GVHD.
Wang, X. et al. Mechanisms of antigen presentation to T cells in murine graft-versus-host disease: cross-presentation and the appearance of cross-presentation. Blood 118, 6426–6437 (2011).
Schultz, K. R., Paquet, J., Bader, S. & HayGlass, K. T. Requirement for B cells in T cell priming to minor histocompatibility antigens and development of graft-versus-host disease. Bone Marrow Transplant. 16, 289–295 (1995).
Knoechel, B., Lohr, J., Kahn, E. & Abbas, A. K. The link between lymphocyte deficiency and autoimmunity: roles of endogenous T and B lymphocytes in tolerance. J. Immunol. 175, 21–26 (2005).
Shimabukuro-Vornhagen, A., Hallek, M. J., Storb, R. F. & von Bergwelt-Baildon, M. S. The role of B cells in the pathogenesis of graft-versus-host disease. Blood 114, 4919–4927 (2009).
Glass, B. et al. 1974 Rituximab for Graft-Versus-Host-Disease-Prophylaxis after Allogeneic Stem Cell Transplantation Given as Treatment of High Risk Relapse of Aggressive Lymphoma: Results of a Randomized Phase II Study. (ASH Annual Meeting and Exposition, 2008).
Kharfan-Dabaja, M. A. & Cutler, C. S. Rituximab for prevention and treatment of graft-versus-host disease. Int. J. Hematol. 93, 578–585 (2011).
Doreau, A. et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nature Immunol. 10, 778–785 (2009).
Zheng, H. et al. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood 111, 2476–2484 (2008).
Cooke, K. R. et al. Tumor necrosis factor-α production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J. Clin. Invest. 102, 1882–1891 (1998).
Nestel, F. P., Price, K. S., Seemayer, T. A. & Lapp, W. S. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor α during graft-versus-host disease. J. Exp. Med. 175, 405–413 (1992).
Reddy, P. Pathophysiology of acute graft-versus-host disease. Hematol. Oncol. 21, 149–161 (2003).
Lu, Y. & Waller, E. K. Dichotomous role of interferon-γ in allogeneic bone marrow transplant. Biol. Blood Marrow Transplant. 15, 1347–1353 (2009).
Brok, H. P., Vossen, J. M. & Heidt, P. J. IFN-γ-mediated prevention of graft-versus-host disease: pharmacodynamic studies and influence on proliferative capacity of chimeric spleen cells. Bone Marrow Transplant. 22, 1005–1010 (1998).
Alousi, A. M. et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood 114, 511–517 (2009). This paper describes a benchmark clinical trial comparing four different therapies for the treatment of steroid-refractory GVHD.
Fowler, D. H. & Gress, R. E. Th2 and Tc2 cells in the regulation of GVHD, GVL, and graft rejection: considerations for the allogeneic transplantation therapy of leukemia and lymphoma. Leuk. Lymphoma 38, 221–234 (2000).
Leveson-Gower, D. B. et al. Low doses of natural killer T cells provide protection from acute graft-versus-host disease via an IL-4-dependent mechanism. Blood 117, 3220–3229 (2011).
Tawara, I. et al. Combined Th2 cytokine deficiency in donor T cells aggravates experimental acute graft-vs-host disease. Exp. Hematol. 36, 988–996 (2008).
Yi, T. et al. Absence of donor Th17 leads to augmented Th1 differentiation and exacerbated acute graft-versus-host disease. Blood 112, 2101–2110 (2008).
Kappel, L. W. et al. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood 113, 945–952 (2009).
Carlson, M. J. et al. In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood 113, 1365–1374 (2009).
Iclozan, C. et al. T helper17 cells are sufficient but not necessary to induce acute graft-versus-host disease. Biol. Blood Marrow Transplant. 16, 170–178 (2010).
Davis, I. D. et al. Interleukin-21 signaling: functions in cancer and autoimmunity. Clin. Cancer Res. 13, 6926–6932 (2007).
Spolski, R., Kashyap, M., Robinson, C., Yu, Z. & Leonard, W. J. IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc. Natl Acad. Sci. USA 105, 14028–14033 (2008).
Peluso, I. et al. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J. Immunol. 178, 732–739 (2007).
Nurieva, R. et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448, 480–483 (2007).
Fantini, M. C. et al. IL-21 regulates experimental colitis by modulating the balance between Treg and Th17 cells. Eur. J. Immunol. 37, 3155–3163 (2007).
Bucher, C. et al. IL-21 blockade reduces graft-versus-host disease mortality by supporting inducible T regulatory cell generation. Blood 114, 5375–5384 (2009).
Hippen, K. L. et al. Blocking IL-21 signaling ameliorates xenogeneic GVHD induced by human lymphocytes. Blood 119, 619–628 (2012).
Hanash, A. M. et al. Abrogation of donor T-cell IL-21 signaling leads to tissue-specific modulation of immunity and separation of GVHD from GVL. Blood 118, 446–455 (2011).
Meguro, A. et al. Lack of IL-21 signal attenuates graft-versus-leukemia effect in the absence of CD8 T-cells. Bone Marrow Transplant. 46, 1557–1565 (2011).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Veldhoen, M., Hocking, R. J., Atkins, C. J., Locksley, R. M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Symington, F. W. et al. The relationship of serum IL-6 levels to acute graft-versus-host disease and hepatorenal disease after human bone marrow transplantation. Transplantation 54, 457–462 (1992).
Ambruzova, Z. et al. Association of IL6 and CCL2 gene polymorphisms with the outcome of allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 44, 227–235 (2009).
Cavet, J. et al. Interferon-γ and interleukin-6 gene polymorphisms associate with graft-versus-host disease in HLA-matched sibling bone marrow transplantation. Blood 98, 1594–1600 (2001).
Chen, X. et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood 114, 891–900 (2009).
Das, R. et al. Blockade of interleukin-23 signaling results in targeted protection of the colon and allows for separation of graft-versus-host and graft-versus-leukemia responses. Blood 115, 5249–5258 (2010).
Williamson, E., Garside, P., Bradley, J. A., More, I. A. & Mowat, A. M. Neutralizing IL-12 during induction of murine acute graft-versus-host disease polarizes the cytokine profile toward a Th2-type alloimmune response and confers long term protection from disease. J. Immunol. 159, 1208–1215 (1997).
Sykes, M. et al. Dose and timing of interleukin (IL)-12 and timing and type of total-body irradiation: effects on graft-vs-host disease inhibition and toxicity of exogenous IL-12 in murine bone marrow transplant recipients. Biol. Blood Marrow Transplant. 5, 277–284 (1999).
Yang, Y. G., Dey, B. R., Sergio, J. J., Pearson, D. A. & Sykes, M. Donor-derived interferon γ is required for inhibition of acute graft-versus-host disease by interleukin 12. J. Clin. Invest. 102, 2126–2135 (1998).
Pidala, J., Perez, L., Beato, F. & Anasetti, C. Ustekinumab demonstrates activity in glucocorticoid-refractory acute GVHD. Bone Marrow Transplant. 29 Aug 2011 (doi:10.1038/bmt.2011.172).
Briones, J., Novelli, S. & Sierra, J. T-cell costimulatory molecules in acute-graft-versus host disease: therapeutic implications. Bone Marrow Res. 2011, 976793 (2011).
Blazar, B. R. et al. CD28/B7 interactions are required for sustaining the graft-versus-leukemia effect of delayed post-bone marrow transplantation splenocyte infusion in murine recipients of myeloid or lymphoid leukemia cells. J. Immunol. 159, 3460–3473 (1997).
Saito, K. et al. Involvement of CD40 ligand–CD40 and CTLA4–B7 pathways in murine acute graft-versus-host disease induced by allogeneic T cells lacking CD28. J. Immunol. 160, 4225–4231 (1998).
Guinan, E. C. et al. Transplantation of anergic histoincompatible bone marrow allografts. N. Engl. J. Med. 340, 1704–1714 (1999).
Taylor, P. A., Noelle, R. J. & Blazar, B. R. CD4+CD25+ immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J. Exp. Med. 193, 1311–1318 (2001).
Sidiropoulos, P. I. & Boumpas, D. T. Lessons learned from anti-CD40L treatment in systemic lupus erythematosus patients. Lupus 13, 391–397 (2004).
Socie, G. & Blazar, B. R. Acute graft-versus-host disease: from the bench to the bedside. Blood 114, 4327–4336 (2009). This review summarizes some of the major advances in our knowledge of the pathophysiology of GVHD and discusses the translation of these advances for the clinical treatment of acute GVHD.
Blazar, B. R. et al. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-γ-dependent mechanism. J. Immunol. 171, 1272–1277 (2003).
Asakura, S. et al. Alloantigen expression on non-hematopoietic cells reduces graft-versus-leukemia effects in mice. J. Clin. Invest. 120, 2370–2378 (2010).
Flutter, B. et al. Nonhematopoietic antigen blocks memory programming of alloreactive CD8+ T cells and drives their eventual exhaustion in mouse models of bone marrow transplantation. J. Clin. Invest. 120, 3855–3868 (2010).
Koestner, W. et al. PD-L1 blockade effectively restores strong graft-versus-leukemia effects without graft-versus-host disease after delayed adoptive transfer of T-cell receptor gene-engineered allogeneic CD8+ T cells. Blood 117, 1030–1041 (2011).
Blazar, B. R., Taylor, P. A., Panoskaltsis-Mortari, A., Sharpe, A. H. & Vallera, D. A. Opposing roles of CD28:B7 and CTLA-4:B7 pathways in regulating in vivo alloresponses in murine recipients of MHC disparate T cells. J. Immunol. 162, 6368–6377 (1999).
Luznik, L. & Fuchs, E. J. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol. Res. 47, 65–77 (2010).
Valenzuela, J. O. et al. PKCθ is required for alloreactivity and GVHD but not for immune responses toward leukemia and infection in mice. J. Clin. Invest. 119, 3774–3786 (2009).
Zanin-Zhorov, A. et al. Protein kinase C-θ mediates negative feedback on regulatory T cell function. Science 328, 372–376 (2010).
Zanin-Zhorov, A., Dustin, M. L. & Blazar, B. R. PKC-θ function at the immunological synapse: prospects for therapeutic targeting. Trends Immunol. 32, 358–363 (2011).
Ihle, J. N. & Kerr, I. M. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 11, 69–74 (1995).
Cetkovic-Cvrlje, M. et al. Treatment of post-bone marrow transplant acute graft-versus-host disease with a rationally designed JAK3 inhibitor. Leuk. Lymphoma 43, 1447–1453 (2002).
Cetkovic-Cvrlje, M., Roers, B. A., Waurzyniak, B., Liu, X. P. & Uckun, F. M. Targeting Janus kinase 3 to attenuate the severity of acute graft-versus-host disease across the major histocompatibility barrier in mice. Blood 98, 1607–1613 (2001).
Betts, B. C. et al. Janus kinase-2 inhibition induces durable tolerance to alloantigen by human dendritic cell-stimulated T cells yet preserves immunity to recall antigen. Blood 118, 5330–5339 (2011).
Olivieri, A. et al. Imatinib for refractory chronic graft-versus-host disease with fibrotic features. Blood 114, 709–718 (2009). This is the first report showing that the tyrosine kinase inhibitor imatinib has clinical efficacy in patients with chronic GVHD.
Stadler, M. et al. Limited efficacy of imatinib in severe pulmonary chronic graft-versus-host disease. Blood 114, 3718–3719 (2009).
Sun, K. et al. Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proc. Natl Acad. Sci. USA 101, 8120–8125 (2004).
Yannaki, E. et al. The proteasome inhibitor bortezomib drastically affects inflammation and bone disease in adjuvant-induced arthritis in rats. Arthritis Rheum. 62, 3277–3288 (2010).
Koreth, J. et al. Bortezomib, tacrolimus, and methotrexate for prophylaxis of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation from HLA-mismatched unrelated donors. Blood 114, 3956–3959 (2009).
Sun, K. et al. Differential effects of proteasome inhibition by bortezomib on murine acute graft-versus-host disease (GVHD): delayed administration of bortezomib results in increased GVHD-dependent gastrointestinal toxicity. Blood 106, 3293–3299 (2005).
Wysocki, C. A. et al. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood 106, 3300–3307 (2005).
Zhou, L., Askew, D., Wu, C. & Gilliam, A. C. Cutaneous gene expression by DNA microarray in murine sclerodermatous graft-versus-host disease, a model for human scleroderma. J. Invest. Dermatol. 127, 281–292 (2007).
Lu, S. X. et al. Absence of P-selectin in recipients of allogeneic bone marrow transplantation ameliorates experimental graft-versus-host disease. J. Immunol. 185, 1912–1919 (2010).
Terwey, T. H. et al. CCR2 is required for CD8-induced graft-versus-host disease. Blood 106, 3322–3330 (2005).
He, S. et al. A new approach to the blocking of alloreactive T cell-mediated graft-versus-host disease by in vivo administration of anti-CXCR3 neutralizing antibody. J. Immunol. 181, 7581–7592 (2008).
Bouazzaoui, A. et al. Steroid treatment alters adhesion molecule and chemokine expression in experimental acute graft-vs-host disease of the intestinal tract. Exp. Hematol. 39, 238–249 (2011).
Inamoto, Y. et al. Donor single nucleotide polymorphism in the CCR9 gene affects the incidence of skin GVHD. Bone Marrow Transplant. 45, 363–369 (2010).
Wysocki, C. A., Panoskaltsis-Mortari, A., Blazar, B. R. & Serody, J. S. Leukocyte migration and graft-versus-host disease. Blood 105, 4191–4199 (2005). This paper reviews the role of leukocyte trafficking in the pathogenesis of GVHD and the potential targets for the treatment of disease.
Waldman, E. et al. Absence of β7 integrin results in less graft-versus-host disease because of decreased homing of alloreactive T cells to intestine. Blood 107, 1703–1711 (2006).
Hashimoto, D. et al. FTY720 enhances the activation-induced apoptosis of donor T cells and modulates graft-versus-host disease. Eur. J. Immunol. 37, 271–281 (2007).
Kim, Y. M., Sachs, T., Asavaroengchai, W., Bronson, R. & Sykes, M. Graft-versus-host disease can be separated from graft-versus-lymphoma effects by control of lymphocyte trafficking with FTY720. J. Clin. Invest. 111, 659–669 (2003).
Taylor, P. A. et al. Insights into the mechanism of FTY720 and compatibility with regulatory T cells for the inhibition of graft-versus-host disease (GVHD). Blood 110, 3480–3488 (2007).
Janssens, W. et al. CD4+CD25+ T cells lyse antigen-presenting B cells by Fas–Fas ligand interaction in an epitope-specific manner. J. Immunol. 171, 4604–4612 (2003).
Piccirillo, C. A. & Shevach, E. M. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin. Immunol. 16, 81–88 (2004).
Serra, P. et al. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity 19, 877–889 (2003).
Buckner, J. H. Mechanisms of impaired regulation by CD4+CD25+FOXP3+ regulatory T cells in human autoimmune diseases. Nature Rev. Immunol. 10, 849–859 (2010).
Cohen, J. L., Trenado, A., Vasey, D., Klatzmann, D. & Salomon, B. L. CD4+CD25+ immunoregulatory T cells: new therapeutics for graft-versus-host disease. J. Exp. Med. 196, 401–406 (2002).
Hoffmann, P., Ermann, J., Edinger, M., Fathman, C. G. & Strober, S. Donor-type CD4+CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J. Exp. Med. 196, 389–399 (2002).
Taylor, P. A., Lees, C. J. & Blazar, B. R. The infusion of ex vivo activated and expanded CD4+CD25+ immune regulatory cells inhibits graft-versus-host disease lethality. Blood 99, 3493–3499 (2002). This is the first study to demonstrate that activated, cultured CD4+CD25+ T cells can offer substantial protection in a relevant in vivo animal model of GVHD.
Gaidot, A. et al. Immune reconstitution is preserved in hematopoietic stem cell transplantation coadministered with regulatory T cells for GVHD prevention. Blood 117, 2975–2983 (2011).
Trenado, A. et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J. Clin. Invest. 112, 1688–1696 (2003).
Edinger, M. et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nature Med. 9, 1144–1150 (2003).
Brunstein, C. G. et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 117, 1061–1070 (2011). This Phase I study was the first to use ex vivo -expanded human allogeneic T Reg cells to prevent GVHD.
Hippen, K. L. et al. Massive ex vivo expansion of human natural regulatory T cells (Tregs) with minimal loss of in vivo functional activity. Sci. Transl. Med. 3, 83ra41 (2011).
Shin, H. J. et al. Rapamycin and IL-2 reduce lethal acute graft-versus-host disease associated with increased expansion of donor type CD4+CD25+Foxp3+ regulatory T cells. Blood 118, 2342–2350 (2011).
Hippen, K. L. et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am. J. Transplant. 11, 1148–1157 (2011).
Sela, U., Olds, P., Park, A., Schlesinger, S. J. & Steinman, R. M. Dendritic cells induce antigen-specific regulatory T cells that prevent graft versus host disease and persist in mice. J. Exp. Med. 208, 2489–2496 (2011).
Semple, K., Yu, Y., Wang, D., Anasetti, C. & Yu, X. Z. Efficient and selective prevention of GVHD by antigen-specific induced Tregs via linked-suppression in mice. Biol. Blood Marrow Transplant. 17, 309–318 (2011).
Sato, K., Yamashita, N., Baba, M. & Matsuyama, T. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity 18, 367–379 (2003).
Waller, E. K. et al. Larger numbers of CD4bright dendritic cells in donor bone marrow are associated with increased relapse after allogeneic bone marrow transplantation. Blood 97, 2948–2956 (2001).
Asai, O. et al. Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. J. Clin. Invest. 101, 1835–1842 (1998). One of the first reports highlighting the role of NK cells in the suppression of GVHD.
Olson, J. A. et al. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood 115, 4293–4301 (2010).
Tanaka, M. et al. The impact of the dose of natural killer cells in the graft on severe acute graft-versus-host disease after unrelated bone marrow transplantation. Leuk. Res. 14 Dec 2011 (doi:10.1016/j.leukres.2011.11.009).
Ruggeri, L. et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295, 2097–2100 (2002).
Miller, J. S. et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood 109, 5058–5061 (2007). A large registry-based study of the role of KIR ligand mismatch in the outcome of haematopoietic cell transplantation for myeloid leukaemias showing less relapse but increased GVHD.
Kohrt, H. E. et al. TLI and ATG conditioning with low risk of graft-versus-host disease retains antitumor reactions after allogeneic hematopoietic cell transplantation from related and unrelated donors. Blood 114, 1099–1109 (2009).
Kuns, R. D. et al. Invariant natural killer T cell–natural killer cell interactions dictate transplantation outcome after α-galactosylceramide administration. Blood 113, 5999–6010 (2009).
Lechner, M. G., Liebertz, D. J. & Epstein, A. L. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J. Immunol. 185, 2273–2284 (2010).
Peranzoni, E. et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr. Opin. Immunol. 22, 238–244 (2010).
Serafini, P., Mgebroff, S., Noonan, K. & Borrello, I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 68, 5439–5449 (2008).
Highfill, S. L. et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood 116, 5738–5747 (2010).
Zhou, Z. et al. Development and function of myeloid-derived suppressor cells generated from mouse embryonic and hematopoietic stem cells. Stem Cells 28, 620–632 (2010).
Aggarwal, S. & Pittenger, M. F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822 (2005).
Polchert, D. et al. IFN-γ activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur. J. Immunol. 38, 1745–1755 (2008).
Sudres, M. et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J. Immunol. 176, 7761–7767 (2006).
Tolar, J., Le Blanc, K., Keating, A. & Blazar, B. R. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells 28, 1446–1455 (2010). This paper reviews the controversies and recent insights in MSC biology, the regulation of alloresponses by MSCs in preclinical models, and clinical experience with MSC infusions and the challenges of this approach.
Kebriaei, P. & Robinson, S. Treatment of graft-versus-host-disease with mesenchymal stromal cells. Cytotherapy 13, 262–268 (2011).
Fallarino, F. & Grohmann, U. Using an ancient tool for igniting and propagating immune tolerance: IDO as an inducer and amplifier of regulatory T cell functions. Curr. Med. Chem. 18, 2215–2221 (2011).
Jasperson, L. K. et al. Indoleamine 2,3-dioxygenase is a critical regulator of acute graft-versus-host disease lethality. Blood 111, 3257–3265 (2008).
Ratajczak, P. et al. IDO in human gut graft-versus-host disease. Biol. Blood Marrow Transplant. 18, 150–155 (2012).
Furuzawa-Carballeda, J. et al. High levels of IDO-expressing CD16+ peripheral cells, and Tregs in graft biopsies from kidney transplant recipients under belatacept treatment. Transplant. Proc. 42, 3489–3496 (2010).
Jasperson, L. K. et al. Inducing the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase (IDO), for suppression of graft-versus-host disease (GVHD) lethality. Blood 114, 5062–5070 (2009).
Zhang, Y. et al. Notch signaling is a critical regulator of allogeneic CD4+ T-cell responses mediating graft-versus-host disease. Blood 117, 299–308 (2011).
Choi, J. et al. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood 116, 129–139 (2010).
Goodyear, O. C. et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia. Blood 119, 3361–3369 (2012).
Bastien, J. P. et al. Photodepletion differentially affects CD4+ Tregs versus CD4+ effector T cells from patients with chronic graft-versus-host disease. Blood 116, 4859–4869 (2010).
Gatza, E. et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood 112, 1515–1521 (2008).
He, S. et al. Inhibition of histone methylation arrests ongoing graft-versus-host disease in mice by selectively inducing apoptosis of alloreactive effector T cells. Blood 119, 1274–1282 (2012).
Choi, S. & Reddy, P. HDAC inhibition and graft versus host disease. Mol. Med. 17, 404–416 (2011).
Reddy, P. et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc. Natl Acad. Sci. USA 101, 3921–3926 (2004).
Lu, S. X. et al. STAT-3 and ERK 1/2 phosphorylation are critical for T-cell alloactivation and graft-versus-host disease. Blood 112, 5254–5258 (2008).
Radojcic, V. et al. STAT3 signaling in CD4+ T cells is critical for the pathogenesis of chronic sclerodermatous graft-versus-host disease in a murine model. J. Immunol. 184, 764–774 (2010).
Ma, H. et al. Absence of Stat1 in donor CD4 T cells promotes the expansion of Tregs and reduces graft-versus-host disease in mice. J. Clin. Invest. 121, 2554–2569 (2011).
Neufert, C. et al. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur. J. Immunol. 37, 1809–1816 (2007).
Goldstein, J. L. & Brown, M. S. Regulation of the mevalonate pathway. Nature 343, 425–430 (1990).
Rotta, M. et al. Impact of recipient statin treatment on graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 16, 1463–1466 (2010).
Di Stasi, A. et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 365, 1673–1683 (2011).
Penack, O., Socie, G. & van den Brink, M. R. The importance of neovascularization and its inhibition for allogeneic hematopoietic stem cell transplantation. Blood 117, 4181–4189 (2011).
Weiden, P. L. et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N. Engl. J. Med. 300, 1068–1073 (1979).
Apperley, J. F. et al. Bone marrow transplantation for chronic myeloid leukaemia in first chronic phase: importance of a graft-versus-leukaemia effect. Br. J. Haematol. 69, 239–245 (1988).
Acknowledgements
We thank the current and past members of our laboratories and our colleagues who have collaborated with us for their scientific contributions, and A. Baptiste for expert technical assistance in the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Allogeneic haematopoietic stem cell transplantation
-
A transplant with allogeneic haematopoietic stem cells (HSCs) is a treatment in which the transplanted HSCs are obtained from a normal donor. This approach can be used to treat either malignant or non-malignant disorders. Mismatches between the histocompatibility antigens of the donor and patient can lead to adverse events, such as rejection of the transplanted graft or pathological immune responses to normal tissues in the patient.
- Conditioning regimen
-
(Also known as a preparative regimen). A combination of chemotherapy, radiation therapy and/or immunosuppressive medications that is designed not only to destroy residual malignant cells, but also to provide space for donor stem cell engraftment and to provide immunosuppression to prevent host rejection of the donor stem cells.
- Myelosuppressive
-
Refers to conditioning regimens that abrogate bone marrow activity, resulting in a marked decrease in the production of blood cells and platelets.
- Non-myeloablative
-
Refers to conditioning regimens that do not fully ablate bone marrow activity, resulting in no decrease (or at most a modest decrease) in the production of blood cells and platelets. Such regimens still help with engraftment and prevent the rejection of donor cells.
- Graft-versus-tumour effects
-
(GVT effects). The antitumour activity of donor T cells against residual malignant cells of the graft recipient following (allogeneic) bone marrow transplantation.
- Minor histocompatibility antigens
-
Normal proteins that are polymorphic in a given population. Even when a transplant donor and recipient are identical with respect to their MHC genes, the amino acid differences in minor proteins can cause graft solid-organ tissue to be slowly rejected.
- Genetic drift
-
The process of change in the genetic composition of a population owing to chance or random events rather than natural selection, resulting in changes in allele frequencies over time.
- Toll-like receptors
-
(TLRs). A family of membrane-spanning proteins that recognize pathogen-associated molecular patterns (which are shared by various microorganisms), as well as damaged host cell components. TLRs signal to the host that a microbial pathogen is present or that tissue damage has occurred. They are characterized by an ectodomain that has varying numbers of leucine-rich repeat motifs and a cytoplasmic Toll/IL-1 receptor (TIR) domain that recruits adaptors, such as myeloid differentiation primary-response protein 88 (MYD88) and TIR domain-containing adaptor protein inducing IFNβ (TRIF; also known as TICAM1).
- Cytokine storm
-
An overproduction of pro-inflammatory mediators in a relatively short period of time in response to the stimulation of T cells, NK cells, monocytes and macrophages by pathogens, tissue injury or other immune insults. Typically, a cytokine storm consists of one or more positive feedback loops between cytokines and immune cells.
- NOD-like receptors
-
(NLRs). The human NLR family comprises 22 members. They share a domain organization that usually includes an amino-terminal caspase-recruitment domain (CARD) or pyrin domain (PYD), followed by an intermediary nucleotide-binding oligomerization domain (NOD) and carboxy-terminal leucine-rich repeat motifs. NLRs are thought to survey the host cytosol and intracellular compartments for pathogen- and damage-associated molecular patterns to activate signalling pathways that contribute to the host innate immune response.
- P2X7
-
An ATP-gated cation channel that is expressed by haematopoietic cells and participates in cell proliferation and apoptosis. It belongs to the family of purinoceptors for ATP and is responsible for the ATP-dependent lysis of macrophages through the formation of membrane pores.
- Inflammasome
-
A large multiprotein complex formed by a NOD-like receptor (NLR), the adaptor protein ASC and pro-caspase 1. The assembly of the inflammasome leads to the activation of caspase 1, which cleaves pro-interleukin-1β (pro-IL-1β) and pro-IL-18 to generate the active pro-inflammatory cytokines.
- Germinal centre
-
A highly specialized and dynamic microenvironment located in peripheral lymphoid tissues (for example, the spleen or lymph nodes). It is the main site of B cell maturation, which leads to the generation of memory B cells and plasma cells that produce high-affinity antibodies.
- TH1 cells
-
(T helper 1 cells). TH1 cells secrete interferon-γ and tumour necrosis factor to promote cell-mediated immunity by supporting the classical activation of macrophages and the proliferation of cytotoxic CD8+ T cells.
- Janus kinases
-
(JAKs). Kinases that are activated downstream of cytokine receptor ligation. Activated JAKs phosphorylate various signal transducer and activator of transcription (STAT) molecules, which in turn translocate to the nucleus, where they transactivate various genes involved in cell differentiation, survival, apoptosis and proliferation.
- Tolerogenic DCs
-
A subpopulation of dendritic cells that can promote antigen-specific peripheral and central tolerance by inducing antigen-specific regulatory T cells, T cell anergy and the deletion of antigen-specific cytotoxic T cells.
- Mixed lymphocyte reaction
-
A tissue-culture technique for testing T cell reactivity and antigen-presenting cell (APC) activity. A population of T cells is cultured with MHC-mismatched APCs, and the proliferation of the T cells is determined by measuring the incorporation of 3H-thymidine into the DNA of dividing cells.
- Invariant NKT cells
-
(iNKT cells). A subset of T cells that possess a semi-invariant T cell receptor. In both mice and humans, iNKT cells recognize ligands presented by CD1d.
- Indoleamine 2,3-dioxygenase
-
(IDO). An intracellular haem-containing enzyme that catalyses the oxidative catabolism of tryptophan. Insufficient availability of tryptophan can lead to T cell apoptosis or anergy.
- Alternatively activated macrophages
-
(Also known as M2 macrophages). Macrophages that are stimulated by interleukin-4 (IL-4) or IL-13 and express arginase 1, the mannose receptor CD206 and IL-4 receptor-α. Pathogen-associated molecular patterns expressed by helminths may also drive the alternative activation of macrophages.
- Mesenchymal stem cells
-
(MSCs; also known as marrow stromal cells). Bone marrow-derived multipotent stem cells that can differentiate into various cell types of mesenchymal origin, including osteoblasts, chondrocytes and adipocytes. MSCs have been shown to have immunomodulatory and immunosuppressive effects.
- Azacitidine
-
A pyrimidine nucleoside analogue of cytidine that inhibits DNA methyltransferase, thereby blocking DNA methylation. The hypomethylation of DNA that results from azacitidine treatment may activate an array of genes, such as tumour suppressor genes that have been silenced by hypermethylation, resulting for example in an antitumour effect. Azacitidine is also incorporated into RNA, thereby disrupting normal RNA function and impairing the activity of tRNA (cytosine-5)-methyltransferase.
- Extracorporeal photopheresis
-
A form of photodynamic therapy in which mononuclear cells are separated from blood by leukopheresis and chemically treated with a photosensitizer such as 8-methoxypsoralen, exposed to ultraviolet light, and re-infused into the patient. The mechanism of action has been proposed to involve alloreactive T cell depletion and TReg cell induction.
- Statins
-
A family of inhibitors of hydroxymethylglutaryl-CoA reductase (HMG-CoA reductase), an enzyme that catalyses the conversion of HMG-CoA to L-mevalonate. Statins are mainly used as cholesterol-lowering drugs, but they also have immunoregulatory and anti-inflammatory properties. L-mevalonate and its metabolites are implicated in cholesterol synthesis and other intracellular pathways.
Rights and permissions
About this article
Cite this article
Blazar, B., Murphy, W. & Abedi, M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol 12, 443–458 (2012). https://doi.org/10.1038/nri3212
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3212