Key Points
-
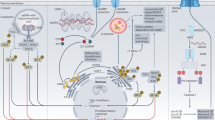
Nucleic acids derived from microorganisms or dying host cells are recognized by Toll-like receptor 7 (TLR7), TLR8 and TLR9 in endo-lysosomal compartments. In the cytoplasm, there are several distinct mechanisms for nucleic acid recognition.
-
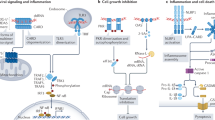
Cytoplasmic DNA from microorganisms or delivered by transfection can trigger two main responses. First, cytoplasmic DNA activates a transcriptional response leading to the activation of nuclear factor-κB (NF-κB) and interferon regulatory factor 3 (IRF3) and the subsequent production of pro-inflammatory cytokines and type I interferons (IFNs). Second, cytoplasmic DNA triggers a proteolytic cascade leading to the activation of interleukin-1β (IL-1β) family cytokines.
-
The transcriptional response to cytoplasmic DNA is triggered by DNA-dependent activator of IRFs (DAI) and potentially other DNA sensors, leading to the initiation of a signalling pathway that involves stimulator of IFN genes (STING) and IRF3. Another DNA-sensing pathway that leads to gene induction is enabled by RNA polymerase III, which transcribes AT-rich double-stranded DNA (dsDNA) into 5′ triphosphate dsRNA. The generated RNA activates the retinoic acid-inducible gene I (RIG-I) pathway. This pathway is functional, but redundant, in mouse cells.
-
Cytoplasmic DNA induces the PYHIN family protein AIM2 (absent in melanoma 2) to form an inflammasome together with ASC (apoptosis-related speck-like protein). The induction of the AIM2 inflammasome leads to the activation of caspase-1, which in turn cleaves and activates pro-IL-1β and pro-IL-18.
-
The signalling pathways induced by cytoplasmic DNA are better understood than the receptors that are involved in activating these pathways. AT-rich dsDNA and dsDNA of random sequence activate partially overlapping and redundant signalling receptors. The identification of other cytoplasmic signalling molecules that are involved in DNA sensing is an important task. A better understanding of intracellular DNA recognition could lead to important targets for the development of therapies for DNA-related immune diseases.
Abstract
The recognition of nucleic acids is one strategy by which cells can detect infectious agents. As life is ultimately determined by the existence of nucleic acids, this defence strategy has evolved in many different organisms and operates effectively in many different cell types. Here, we review the recent progress in our understanding of the molecular mechanisms by which DNA activates cells to induce inflammation and antimicrobial immunity. DNA can be detected in different cellular compartments and can induce a range of cellular responses, such as an antiviral response and pyroptotic cell death together with the maturation and release of active interleukin-1β.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003).
Takeuchi, O. & Akira, S. MDA5/RIG-I and virus recognition. Curr. Opin. Immunol. 20, 17–22 (2008).
Isaacs, A., Cox, R. A. & Rotem, Z. Foreign nucleic acids as the stimulus to make interferon. Lancet 2, 113–116 (1963).
Rotem, Z., Cox, R. A. & Isaacs, A. Inhibition of virus multiplication by foreign nucleic acid. Nature 197, 564–566 (1963).
Lampson, G. P., Tytell, A. A., Field, A. K., Nemes, M. M. & Hilleman, M. R. Inducers of interferon and host resistance. I. Double-stranded RNA from extracts of Penicillium funiculosum. Proc. Natl Acad. Sci. USA 58, 782–789 (1967).
Tokunaga, T. et al. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity. J. Natl Cancer Inst. 72, 955–962 (1984).
Tokunaga, T. et al. Synthetic oligonucleotides with particular base sequences from the cDNA encoding proteins of Mycobacterium bovis BCG induce interferons and activate natural killer cells. Microbiol. Immunol. 36, 55–66 (1992).
Yamamoto, S. et al. Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN [correction of INF] and augment IFN-mediated [correction of INF] natural killer activity. J. Immunol. 148, 4072–4076 (1992). This study identifies self-palindromic CpG-rich DNA as the IFN-inducing activity in mycobacterial DNA extracts.
Krieg, A. M. et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374, 546–549 (1995). This paper describes that the higher frequency of non-methylated CpG motifs in bacterial DNA compared with mammalian DNA could explain the previously reported effects of bacterial DNA on immune cells.
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000). This study reports the identification of TLR9 as the receptor for extracellular DNA.
Fox, B. A. et al. Lipofection indirectly increases expression of endogenous major histocompatibility complex class I molecules on tumor cells. Cancer Gene Ther. 5, 307–312 (1998).
Park, J. H. et al. Up-regulation of the expression of major histocompatibility complex class I antigens by plasmid DNA transfection in non-hematopoietic cells. FEBS Lett. 436, 55–60 (1998).
Suzuki, K. et al. Activation of target-tissue immune-recognition molecules by double-stranded polynucleotides. Proc. Natl Acad. Sci. USA 96, 2285–2290 (1999).
Stacey, K. J., Ross, I. L. & Hume, D. A. Electroporation and DNA-dependent cell death in murine macrophages. Immunol. Cell Biol. 71, 75–85 (1993).
Okabe, Y., Kawane, K., Akira, S., Taniguchi, T. & Nagata, S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J. Exp. Med. 202, 1333–1339 (2005).
Boule, M. W. et al. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin–immunoglobulin G complexes. J. Exp. Med. 199, 1631–1640 (2004).
Decker, P., Singh-Jasuja, H., Haager, S., Kotter, I. & Rammensee, H. G. Nucleosome, the main autoantigen in systemic lupus erythematosus, induces direct dendritic cell activation via a MyD88-independent pathway: consequences on inflammation. J. Immunol. 174, 3326–3334 (2005).
Yasuda, K. et al. Endosomal translocation of vertebrate DNA activates dendritic cells via TLR9-dependent and -independent pathways. J. Immunol. 174, 6129–6136 (2005).
Ishii, K. J. et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nature Immunol. 7, 40–48 (2006). This study characterizes the signalling pathway triggered by cytoplasmic dsDNA and poly(dA:dT).
Stetson, D. B. & Medzhitov, R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 24, 93–103 (2006). This paper describes the signalling intermediates that are activated by cytoplasmic 45mer IFN-stimulatory dsDNA.
Jin, L. et al. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol. Cell Biol. 28, 5014–5026 (2008).
Zhong, B. et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29, 538–550 (2008).
Zhong, B. et al. The ubiquitin ligase RNF5 regulates antiviral responses by mediating degradation of the adaptor protein MITA. Immunity 30, 397–407 (2009).
Ishikawa, H. & Barber, G. N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678 (2008). This paper shows that STING is a key adaptor protein for cytoplasmic DNA-sensing pathways.
Ishikawa, H., Ma, Z. & Barber, G. N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792 (2009).
Sun, W. et al. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc. Natl Acad. Sci. USA 106, 8653–8658 (2009).
Takaoka, A. et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448, 501–505 (2007). This study identifies the first candidate intracellular DNA receptor for B-DNA.
Ishii, K. J. et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature 451, 725–729 (2008).
Wang, Z. et al. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proc. Natl Acad. Sci. USA 105, 5477–5482 (2008).
Kumar, H. et al. Essential role of IPS-1 in innate immune responses against RNA viruses. J. Exp. Med. 203, 1795–1803 (2006).
Sun, Q. et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity 24, 633–642 (2006).
Cheng, G., Zhong, J., Chung, J. & Chisari, F. V. Double-stranded DNA and double-stranded RNA induce a common antiviral signaling pathway in human cells. Proc. Natl Acad. Sci. USA 104, 9035–9040 (2007).
Ablasser, A. et al. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nature Immunol. 10, 1065–1072 (2009).
Chiu, Y. H., Macmillan, J. B. & Chen, Z. J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138, 576–591 (2009). References 34 and 35 describe that RNA polymerase III transcribes DNA into immune-stimulatory RNA, which activates the RIG-I pathway.
Huet, J., Riva, M., Sentenac, A. & Fromageot, P. Yeast RNA polymerase C and its subunits. Specific antibodies as structural and functional probes. J. Biol. Chem. 260, 15304–15310 (1985).
Zaros, C. & Thuriaux, P. Rpc25, a conserved RNA polymerase III subunit, is critical for transcription initiation. Mol. Microbiol. 55, 104–114 (2005).
Jaehning, J. A. & Roeder, R. G. Transcription of specific adenovirus genes in isolated nuclei by exogenous RNA polymerases. J. Biol. Chem. 252, 8753–8761 (1977).
Samanta, M., Iwakiri, D., Kanda, T., Imaizumi, T. & Takada, K. EB virus-encoded RNAs are recognized by RIG-I and activate signaling to induce type I IFN. EMBO J. 25, 4207–4214 (2006).
Cerretti, D. P. et al. Molecular characterization of the gene for human interleukin-1β converting enzyme (IL1BC). Genomics 20, 468–473 (1994).
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 (2002).
Muruve, D. A. et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452, 103–107 (2008).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Fernandes-Alnemri, T., Yu, J. W., Datta, P., Wu, J. & Alnemri, E. S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009).
Burckstummer, T. et al. An orthogonal proteomic–genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nature Immunol. 10, 266–272 (2009).
Roberts, T. L. et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323, 1057–1060 (2009). References 43–46 define the AIM2 inflammasome that forms in response to cytoplasmic dsDNA.
Choubey, D. & Panchanathan, R. Interferon-inducible Ifi200-family genes in systemic lupus erythematosus. Immunol. Lett. 119, 32–41 (2008).
Pisetsky, D. S. The role of innate immunity in the induction of autoimmunity. Autoimmun. Rev. 8, 69–72 (2008).
Yasutomo, K. et al. Mutation of DNASE1 in people with systemic lupus erythematosus. Nature Genet. 28, 313–314 (2001).
Napirei, M. et al. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nature Genet. 25, 177–181 (2000).
Yoshida, H., Okabe, Y., Kawane, K., Fukuyama, H. & Nagata, S. Lethal anemia caused by interferon-β produced in mouse embryos carrying undigested DNA. Nature Immunol. 6, 49–56 (2005).
Okabe, Y., Kawane, K. & Nagata, S. IFN regulatory factor (IRF) 3/7-dependent and -independent gene induction by mammalian DNA that escapes degradation. Eur. J. Immunol. 38, 3150–3158 (2008).
Stetson, D. B., Ko, J. S., Heidmann, T. & Medzhitov, R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 134, 587–598 (2008). This study describes a mechanism by which cells prevent the development of autoimmunity towards endogenous retroelements through the activity of the exonuclease TREX1.
Crow, Y. J. et al. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutières syndrome at the AGS1 locus. Nature Genet. 38, 917–920 (2006).
Morita, M. et al. Gene-targeted mice lacking the Trex1 (DNase III) 3′-5′ DNA exonuclease develop inflammatory myocarditis. Mol. Cell Biol. 24, 6719–6727 (2004).
Acknowledgements
This work was supported by grants to V.H. from the German Research Foundation (SFB704) and the European Research Council (ERC-2009-StG 243046) and to E.L. from the US National Institutes of Health (AI-065483 and AI-083713) and the Dana Foundation.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Glossary
- Type I interferons
-
(IFNs). Type I IFNs bind to the IFNα receptor (IFNAR) cell surface complex, which consists of IFNAR1 and IFNAR2 chains. The type I IFNs in humans are mainly IFNα, IFNβ and IFNω and these IFNs mediate the inhibition of viral replication, activate natural killer cells and macrophages and increase antigen presentation to T cells during infections and during an immune response to tumour cells.
- Retroelements
-
Retroelements (also known as retrotransposons) are transposable genetic elements that mediate their transposition life cycle through a process that involves the reverse transcription of RNA to DNA. Three main groups of retroelements exist in the human genome: long interspersed nuclear elements (LINEs; which are autonomous retrotransposons), small interspersed nuclear elements (SINEs) and long terminal repeat (LTR) retrotransposons. In the human genome, a few LINE retrotransposons (L1s) are active and can move their own and SINE sequences into different genomic locations. SINEs are nonautonomous retrotransposons that do not encode reverse transcriptase activity and therefore require LINEs for their propagation.
- Systemic lupus erythematosus
-
(SLE). This is a prototypical nucleic acid-triggered autoimmune disease. High titres of autoantibodies specific for nucleoproteins, DNA and RNA can be found in the sera of patients with SLE and these autoantibodies induce immune-mediated tissue inflammation and damage.
- RNA polymerase III
-
In eukaryotes, transcription is carried out by three distinct DNA-dependent RNA polymerases, known as RNA polymerase I, RNA polymerase II and RNA polymerase III. These RNA polymerases are multisubunit enzyme complexes that share a structurally conserved core, but they differ in the composition of subunits located at the outer part of the transcription complex. RNA polymerase III transcribes non-coding RNAs including, but not limited to, transfer RNAs, ribosomal 5S rRNA, small nuclear RNAs, small nucleolar RNAs, microRNAs and Piwi-interacting RNAs.
- IFN regulatory factor
-
(IRF). A transcription factor involved in the regulation of IFN gene transcription. IRF3 and IRF7 have both been implicated downstream of the recognition pathways for intracellular DNA.
- TANK-binding kinase 1
-
(TBK1). A serine/threonine kinase that phosphorylates and thereby activates IRF3 and IRF7, allowing their nuclear translocation. This leads to the production of type I IFNs.
- Pyroptosis
-
A form of cell death that is triggered concomitantly with the activation of the inflammasome and requires caspase-1 activity. During pyroptotic cell death, one single large supramolecular complex consisting of ASC and eventually other inflammasome components forms to activate caspase-1.
Rights and permissions
About this article
Cite this article
Hornung, V., Latz, E. Intracellular DNA recognition. Nat Rev Immunol 10, 123–130 (2010). https://doi.org/10.1038/nri2690
Issue Date:
DOI: https://doi.org/10.1038/nri2690
This article is cited by
-
Pattern recognition receptors and their nano-adjuvants for cancer immunotherapy
Journal of Pharmaceutical Investigation (2023)
-
Global profiling reveals common and distinct N6-methyladenosine (m6A) regulation of innate immune responses during bacterial and viral infections
Cell Death & Disease (2022)
-
The delivery challenge: fulfilling the promise of therapeutic genome editing
Nature Biotechnology (2020)
-
Rationale of combination of anti-PD-1/PD-L1 antibody therapy and radiotherapy for cancer treatment
International Journal of Clinical Oncology (2020)
-
Progressive waves of IL-1β release by primary human monocytes via sequential activation of vesicular and gasdermin D-mediated secretory pathways
Cell Death & Disease (2018)