Key Points
-
HIV infection leads to perturbations of all of the main cell types of the immune system, including B cells. Most B-cell perturbations are associated with indirect effects of ongoing HIV replication, although some perturbations arise regardless of the decrease in viraemia by effective antiretroviral therapy (ART).
-
Many B-cell defects in HIV infection are associated with alterations in the B-cell subpopulations that circulate in the peripheral blood. Most B cells in healthy individuals comprise resting naive and memory B cells, whereas several minor subpopulations are over-represented in HIV-infected individuals, including immature transitional B cells, exhausted B cells, activated mature B cells and plasmablasts.
-
In vitro and in vivo studies show that HIV can also interact directly with B cells, although the frequency of these interactions is unlikely to account for the extent of B-cell dysregulation that is observed in infected individuals. HIV virions complexed with complement and antibody can bind B cells through the complement receptor CD21, and HIV virions and proteins, including gp120 and Nef, can also interact directly or indirectly with B cells.
-
Indirect effects of HIV viraemia on B cells include B-cell hyperactivity (as manifested by hypergammaglobulinaemia), increased B-cell polyclonal activation, increased B-cell turnover, increased expression of activation markers and of markers that are associated with activation-induced apoptosis, increased frequency of B-cell malignancies and increased differentiation of B cells to plasmablasts.
-
HIV viraemia with CD4+ T-cell lymphopenia is associated with an increased frequency of immature transitional B cells. This increase is also associated with increased serum levels of interleukin-7.
-
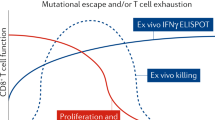
HIV viraemia leads to B-cell exhaustion, as manifested by increased expression of multiple inhibitory receptors, altered expression of homing receptors, decreased cell division and somatic hypermutation in vivo, decreased proliferative and effector properties in vitro and enrichment of HIV-specific responses in the exhausted B-cell compartment.
-
Although most B-cell perturbations in HIV-infected individuals are attributed to viraemia and are reversible by ART, one important exception is the loss of memory B cells. All stages of HIV infection are associated with a decrease both in the frequency of resting memory B cells and of B-cell responses against T-cell-dependent and T-cell-independent antigens.
-
Many B-cell perturbations observed in HIV infection also arise in various infectious and non-infectious disease settings that involve immune dysfunction. Several human diseases that affect B cells show dysregulation of immature transitional B cells, whereas others show dysregulation of memory B cells, with alterations that are similar to B-cell exhaustion and activation-induced terminal differentiation.
Abstract
In recent years, intense research efforts have been dedicated to elucidating the pathogenic mechanisms of HIV-associated disease progression. In addition to the progressive depletion and dysfunction of CD4+ T cells, HIV infection also leads to extensive defects in the humoral arm of the immune system. The lack of immune control of the virus in almost all infected individuals is a great impediment to the treatment of HIV-associated disease and to the development of a successful HIV vaccine. This Review focuses on advances in our understanding of the mechanisms of B-cell dysfunction in HIV-associated disease and discusses similarities with other diseases that are associated with B-cell dysfunction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lane, H. C. et al. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 309, 453–458 (1983). This study was the first to describe B-cell hyperactivity and dysfunction in individuals with AIDS.
Barre-Sinoussi, F. et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220, 868–871 (1983).
Popovic, M., Sarngadharan, M. G., Read, E. & Gallo, R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224, 497–500 (1984).
Grossman, Z., Meier-Schellersheim, M., Paul, W. E. & Picker, L. J. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nature Med. 12, 289–295 (2006).
Sodora, D. L. & Silvestri, G. Immune activation and AIDS pathogenesis. AIDS 22, 439–446 (2008).
Ammann, A. J. et al. B-cell immunodeficiency in acquired immune deficiency syndrome. JAMA 251, 1447–1449 (1984).
Pahwa, S. G., Quilop, M. T., Lange, M., Pahwa, R. N. & Grieco, M. H. Defective B-lymphocyte function in homosexual men in relation to the acquired immunodeficiency syndrome. Ann. Intern. Med. 101, 757–763 (1984).
Carbone, A. et al. Lymph node immunohistology in intravenous drug abusers with persistent generalized lymphadenopathy. Arch. Pathol. Lab. Med. 109, 1007–1012 (1985).
Kekow, J., Kern, P., Schmitz, H. & Gross, W. L. Abnormal B-cell response to T-cell-independent polyclonal B-cell activators in homosexuals presenting persistent generalized lymph node enlargement and HTLV-III antibodies. Diagn. Immunol. 4, 107–111 (1986).
Schnittman, S. M., Lane, H. C., Higgins, S. E., Folks, T. & Fauci, A. S. Direct polyclonal activation of human B lymphocytes by the acquired immune deficiency syndrome virus. Science 233, 1084–1086 (1986).
Kacani, L. et al. Detachment of human immunodeficiency virus type 1 from germinal centers by blocking complement receptor type 2. J. Virol. 74, 7997–8002 (2000).
Moir, S. et al. B cells of HIV-1-infected patients bind virions through CD21-complement interactions and transmit infectious virus to activated T cells. J. Exp. Med. 192, 637–646 (2000).
Malaspina, A. et al. Human immunodeficiency virus type 1 bound to B cells: relationship to virus replicating in CD4+ T cells and circulating in plasma. J. Virol. 76, 8855–8863 (2002).
Thacker, T. C. et al. Follicular dendritic cells and human immunodeficiency virus type 1 transcription in CD4+ T cells. J. Virol. 83, 150–158 (2009).
Shen, L. & Siliciano, R. F. Viral reservoirs, residual viremia, and the potential of highly active antiretroviral therapy to eradicate HIV infection. J. Allergy Clin. Immunol. 122, 22–28 (2008).
Rappocciolo, G. et al. DC-SIGN on B lymphocytes is required for transmission of HIV-1 to T lymphocytes. PLoS Pathog. 2, e70 (2006).
He, B. et al. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J. Immunol. 176, 3931–3941 (2006).
Berberian, L., Goodglick, L., Kipps, T. J. & Braun, J. Immunoglobulin VH3 gene products: natural ligands for HIV gp120. Science 261, 1588–1591 (1993).
Moir, S. et al. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc. Natl Acad. Sci. USA 98, 10362–10367 (2001). By identifying terminally differentiated CD21low B cells in HIV-viraemic individuals, the authors provide insight into the hypergammaglobulinaemia that is associated with HIV disease.
Notermans, D. W. et al. Potent antiretroviral therapy initiates normalization of hypergammaglobulinemia and a decline in HIV type 1-specific antibody responses. AIDS Res. Hum. Retroviruses 17, 1003–1008 (2001).
Shearer, W. T. et al. Prospective 5-year study of peripheral blood CD4, CD8, and CD19/CD20 lymphocytes and serum Igs in children born to HIV-1 women. J. Allergy Clin. Immunol. 106, 559–566 (2000).
Shirai, A., Cosentino, M., Leitman-Klinman, S. F. & Klinman, D. M. Human immunodeficiency virus infection induces both polyclonal and virus-specific B cell activation. J. Clin. Invest. 89, 561–566 (1992). This study provides the first comprehensive analysis of HIV-induced B-cell hyperactivity at the cellular level.
Moir, S. et al. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J. Exp. Med. 200, 587–599 (2004).
Kovacs, J. A. et al. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. J. Exp. Med. 194, 1731–1741 (2001).
Malaspina, A. et al. Deleterious effect of HIV-1 plasma viremia on B cell costimulatory function. J. Immunol. 170, 5965–5972 (2003).
De Milito, A. et al. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood 103, 2180–2186 (2004).
Martinez-Maza, O., Crabb, E., Mitsuyasu, R. T., Fahey, J. L. & Giorgi, J. V. Infection with the human immunodeficiency virus (HIV) is associated with an in vivo increase in B lymphocyte activation and immaturity. J. Immunol. 138, 3720–3724 (1987).
van der Meijden, M. et al. IL-6 receptor (CD126′IL-6R′) expression is increased on monocytes and B lymphocytes in HIV infection. Cell Immunol. 190, 156–166 (1998).
Nagase, H. et al. Mechanism of hypergammaglobulinemia by HIV infection: circulating memory B-cell reduction with plasmacytosis. Clin. Immunol. 100, 250–259 (2001).
Conge, A. M. et al. Impairment of B-lymphocyte differentiation induced by dual triggering of the B-cell antigen receptor and CD40 in advanced HIV-1-disease. AIDS 12, 1437–1449 (1998).
Haynes, B. F. et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 308, 1906–1908 (2005).
Ng, V. L. B-lymphocytes and autoantibody profiles in HIV disease. Clin. Rev. Allergy Immunol. 14, 367–384 (1996).
Martinez-Maza, O. & Breen, E. C. B-cell activation and lymphoma in patients with HIV. Curr. Opin. Oncol. 14, 528–532 (2002).
Mandl, J. N. et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nature Med. 14, 1077–1087 (2008).
Rieckmann, P., Poli, G., Fox, C. H., Kehrl, J. H. & Fauci, A. S. Recombinant gp120 specifically enhances tumor necrosis factor-alpha production and Ig secretion in B lymphocytes from HIV-infected individuals but not from seronegative donors. J. Immunol. 147, 2922–2927 (1991).
Weimer, R. et al. HIV-induced IL-6/IL-10 dysregulation of CD4 cells is associated with defective B cell help and autoantibody formation against CD4 cells. Clin. Exp. Immunol. 111, 20–29 (1998).
Muller, F., Aukrust, P., Nordoy, I. & Froland, S. S. Possible role of interleukin-10 (IL-10) and CD40 ligand expression in the pathogenesis of hypergammaglobulinemia in human immunodeficiency virus infection: modulation of IL-10 and Ig production after intravenous Ig infusion. Blood 92, 3721–3729 (1998).
Diop, O. M. et al. Plasmacytoid dendritic cell dynamics and α interferon production during Simian immunodeficiency virus infection with a nonpathogenic outcome. J. Virol. 82, 5145–5152 (2008).
Brenchley, J. M. et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature Med. 12, 1365–1371 (2006). References 37 and 39 indicate potential mediators of B-cell hyperactivity in HIV-infected individuals with chronic viraemia.
Brenchley, J. M., Price, D. A. & Douek, D. C. HIV disease: fallout from a mucosal catastrophe? Nature Immunol. 7, 235–239 (2006).
Wagner, M. et al. IL-12p70-dependent Th1 induction by human B cells requires combined activation with CD40 ligand and CpG DNA. J. Immunol. 172, 954–963 (2004).
Swingler, S. et al. HIV-1 Nef intersects the macrophage CD40L signalling pathway to promote resting-cell infection. Nature 424, 213–219 (2003).
Swingler, S. et al. Evidence for a pathogenic determinant in HIV-1 Nef involved in B cell dysfunction in HIV/AIDS. Cell Host Microbe 4, 63–76 (2008).
Qiao, X. et al. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nature Immunol. 7, 302–310 (2006). References 42–44 identify potential mechanisms of Nef-mediated B-cell hyperactivity and dysfunction in HIV-infected individuals.
Giri, M. S., Nebozhyn, M., Showe, L. & Montaner, L. J. Microarray data on gene modulation by HIV-1 in immune cells: 2000–2006. J. Leukoc. Biol. 80, 1031–1043 (2006).
Napolitano, L. A. et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nature Med. 7, 73–79 (2001).
Fry, T. J. et al. A potential role for interleukin-7 in T-cell homeostasis. Blood 97, 2983–2990 (2001).
LeBien, T. W. & Tedder, T. F. B lymphocytes: how they develop and function. Blood 112, 1570–1580 (2008).
Malaspina, A. et al. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease: correlation with increased IL-7. Proc. Natl Acad. Sci. USA 103, 2262–2267 (2006).
Malaspina, A. et al. Idiopathic CD4+ T lymphocytopenia is associated with increases in immature/transitional B cells and serum levels of IL-7. Blood 109, 2086–2088 (2007).
Moir, S. & Fauci, A. S. Pathogenic mechanisms of B-lymphocyte dysfunction in HIV disease. J. Allergy Clin. Immunol. 122, 12–19 (2008).
Moir, S. et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 205, 1797–1805 (2008).
Gallimore, A. et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I–peptide complexes. J. Exp. Med. 187, 1383–1393 (1998).
Day, C. L. et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443, 350–354 (2006).
Trautmann, L. et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nature Med. 12, 1198–1202 (2006).
Kaufmann, D. E. et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nature Immunol. 8, 1246–1254 (2007).
Ehrhardt, G. R. et al. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J. Exp. Med. 202, 783–791 (2005).
Ehrhardt, G. R. et al. Discriminating gene expression profiles of memory B cell subpopulations. J. Exp. Med. 205, 1807–1817 (2008). References 52, 57 and 58 describe distinct memory B cells that might have immunoregulatory roles in health and disease.
Wherry, E. J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Klein, U. & Dalla-Favera, R. Germinal centres: role in B-cell physiology and malignancy. Nature Rev. Immunol. 8, 22–33 (2008).
Cagigi, A. et al. Altered expression of the receptor-ligand pair CXCR5/CXCL13 in B-cells during chronic HIV-1 infection. Blood 112, 4401–4410 (2008).
Shin, H. & Wherry, E. J. CD8 T cell dysfunction during chronic viral infection. Curr. Opin. Immunol. 19, 408–415 (2007).
Morris, L. et al. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J. Exp. Med. 188, 233–245 (1998). This study was the first to describe changes in B-cell responses after effective ART.
Nilssen, D. E., Oktedalen, O. & Brandtzaeg, P. Intestinal B cell hyperactivity in AIDS is controlled by highly active antiretroviral therapy. Gut 53, 487–493 (2004).
Fournier, A. M. et al. Dynamics of spontaneous HIV-1 specific and non-specific B-cell responses in patients receiving antiretroviral therapy. AIDS 16, 1755–1760 (2002).
Jacobson, M. A., Khayam-Bashi, H., Martin, J. N., Black, D. & Ng, V. Effect of long-term highly active antiretroviral therapy in restoring HIV-induced abnormal B-lymphocyte function. J. Acquir. Immune Defic. Syndr. 31, 472–477 (2002).
Horvath, A. et al. High level of anticholesterol antibodies (ACHA) in HIV patients. Normalization of serum ACHA concentration after introduction of HAART. Immunobiology 203, 756–768 (2001).
Malaspina, A. et al. CpG oligonucleotides enhance proliferative and effector responses of B cells in HIV-infected individuals. J. Immunol. 181, 1199–1206 (2008).
Jiang, W. et al. Impaired naive and memory B-cell responsiveness to TLR9 stimulation in human immunodeficiency virus infection. J. Virol. 82, 7837–7845 (2008).
Moir, S. et al. Perturbations in B cell responsiveness to CD4+ T cell help in HIV-infected individuals. Proc. Natl Acad. Sci. USA 100, 6057–6062 (2003).
De Boer, R. J., Mohri, H., Ho, D. D. & Perelson, A. S. Turnover rates of B cells, T cells, and NK cells in simian immunodeficiency virus-infected and uninfected rhesus macaques. J. Immunol. 170, 2479–2487 (2003).
Silvestri, G., Paiardini, M., Pandrea, I., Lederman, M. M. & Sodora, D. L. Understanding the benign nature of SIV infection in natural hosts. J. Clin. Invest. 117, 3148–3154 (2007).
Kaur, A. et al. Dynamics of T- and B-lymphocyte turnover in a natural host of simian immunodeficiency virus. J. Virol. 82, 1084–1093 (2008).
Ribeiro, R. M. Dynamics of CD4+ T cells in HIV-1 infection. Immunol. Cell Biol. 85, 287–294 (2007).
Bekker, V. et al. Epstein–Barr virus infects B and non-B lymphocytes in HIV-1-infected children and adolescents. J. Infect. Dis. 194, 1323–1330 (2006).
Moir, S. et al. Normalization of B cell counts and subpopulations following antiretroviral therapy in chronic HIV disease. J. Infect. Dis. 197, 572–579 (2008).
Meira, D. G., Lorand-Metze, I., Toro, A. D., Silva, M. T. & Vilela, M. M. Bone marrow features in children with HIV infection and peripheral blood cytopenias. J. Trop. Pediatr. 51, 114–119 (2005).
Le Guillou-Guillemette, H. et al. Immune restoration under HAART in patients chronically infected with HIV-1: diversity of T, B, and NK immune responses. Viral Immunol. 19, 267–276 (2006).
Lederman, M. M. Immune restoration and CD4+ T-cell function with antiretroviral therapies. AIDS 15, S11–S15 (2001).
Gougeon, M. L. et al. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J. Immunol. 156, 3509–3520 (1996).
Ho, J. et al. Two overrepresented B cell populations in HIV-infected individuals undergo apoptosis by different mechanisms. Proc. Natl Acad. Sci. USA 103, 19436–19441 (2006).
Herbeuval, J. P. & Shearer, G. M. HIV-1 immunopathogenesis: how good interferon turns bad. Clin. Immunol. 123, 121–128 (2007).
Titanji, K. et al. Primary HIV-1 infection sets the stage for important B lymphocyte dysfunctions. AIDS 19, 1947–1955 (2005).
Titanji, K. et al. Low frequency of plasma nerve-growth factor detection is associated with death of memory B lymphocytes in HIV-1 infection. Clin. Exp. Immunol. 132, 297–303 (2003).
Chong, Y. et al. Selective CD27+ (memory) B cell reduction and characteristic B cell alteration in drug-naive and HAART-treated HIV type 1-infected patients. AIDS Res. Hum. Retroviruses 20, 219–226 (2004).
De Milito, A. B lymphocyte dysfunctions in HIV infection. Curr. HIV Res. 2, 11–21 (2004).
Cagigi, A., Nilsson, A., De Milito, A. & Chiodi, F. B cell immunopathology during HIV-1 infection: lessons to learn for HIV-1 vaccine design. Vaccine 26, 3016–3025 (2008).
Tarlinton, D. B-cell memory: are subsets necessary? Nature Rev. Immunol. 6, 785–790 (2006).
D'Orsogna, L. J., Krueger, R. G., McKinnon, E. J. & French, M. A. Circulating memory B-cell subpopulations are affected differently by HIV infection and antiretroviral therapy. AIDS 21, 1747–1752 (2007).
Jacobsen, M. C. et al. Pediatric human immunodeficiency virus infection and circulating IgD+ memory B cells. J. Infect. Dis. 198, 481–485 (2008).
De Milito, A., Morch, C., Sonnerborg, A. & Chiodi, F. Loss of memory (CD27) B lymphocytes in HIV-1 infection. AIDS 15, 957–964 (2001).
Hart, M. et al. Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J. Immunol. 178, 8212–8220 (2007).
Malaspina, A. et al. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J. Infect. Dis. 191, 1442–1450 (2005).
Titanji, K. et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood 108, 1580–1587 (2006). This study shows the early and sustained impairment of memory B-cell responses in HIV-infected individuals.
Tejiokem, M. C. et al. HIV-infected children living in central Africa have low persistence of antibodies to vaccines used in the expanded program on immunization. PLoS ONE 2, e1260 (2007).
Kroon, F. P. et al. Restored humoral immune response to influenza vaccination in HIV-infected adults treated with highly active antiretroviral therapy. AIDS 12, F217–F223 (1998).
Kruetzmann, S. et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J. Exp. Med. 197, 939–945 (2003).
Martin, F. & Kearney, J. F. Marginal-zone B cells. Nature Rev. Immunol 2, 323–335 (2002).
Bliss, S. J. et al. The evidence for using conjugate vaccines to protect HIV-infected children against pneumococcal disease. Lancet Infect. Dis. 8, 67–80 (2008).
Meffre, E. et al. Circulating human B cells that express surrogate light chains and edited receptors. Nature Immunol. 1, 207–213 (2000).
Sims, G. P. et al. Identification and characterization of circulating human transitional B cells. Blood 105, 4390–4398 (2005).
Cuss, A. K. et al. Expansion of functionally immature transitional B cells is associated with human-immunodeficient states characterized by impaired humoral immunity. J. Immunol. 176, 1506–1516 (2006).
Anolik, J. H. et al. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis. Rheum. 56, 3044–3056 (2007).
Stohl, W. B lymphocyte stimulator protein levels in systemic lupus erythematosus and other diseases. Curr. Rheumatol. Rep. 4, 345–350 (2002).
Wardemann, H. et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003).
Charles, E. D. et al. Clonal expansion of immunoglobulin M+CD27+ B cells in HCV-associated mixed cryoglobulinemia. Blood 111, 1344–1356 (2008).
Potter, K. N. et al. Disturbances in peripheral blood B cell subpopulations in autoimmune patients. Lupus 11, 872–877 (2002).
Bennett, L. et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197, 711–723 (2003).
Odendahl, M. et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J. Immunol. 165, 5970–5979 (2000).
Wehr, C. et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood 111, 77–85 (2008).
Wei, C. et al. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J. Immunol. 178, 6624–6633 (2007).
Velu, V. et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 10 Dec 2008 (doi:10.1038./nature07662).
Miller, C. J. et al. Antiviral antibodies are necessary for control of simian immunodeficiency virus replication. J. Virol. 81, 5024–5035 (2007).
Gaufin, T. et al. Limited ability of humoral immune responses in control of viremia during infection with SIVsmmD215 strain. Blood 23 Jan 2009 (doi:10.1182/blood-2008-09-177741).
Richman, D. D., Wrin, T., Little, S. J. & Petropoulos, C. J. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl Acad. Sci. USA 100, 4144–4149 (2003).
Wei, X. et al. Antibody neutralization and escape by HIV-1. Nature 422, 307–312 (2003).
Mestecky, J. et al. Paucity of antigen-specific IgA responses in sera and external secretions of HIV-type 1-infected individuals. AIDS Res. Hum. Retroviruses 20, 972–988 (2004).
Scamurra, R. W. et al. Mucosal plasma cell repertoire during HIV-1 infection. J. Immunol. 169, 4008–4016 (2002).
Shattock, R. J., Haynes, B. F., Pulendran, B., Flores, J. & Esparza, J. Improving defences at the portal of HIV entry: mucosal and innate immunity. PLoS Med. 5, e81 (2008).
Lopes-Carvalho, T. & Kearney, J. F. Marginal zone B cell physiology and disease. Curr. Dir. Autoimmun. 8, 91–123 (2005).
Karlsson Hedestam, G. B. et al. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nature Rev. Microbiol. 6, 143–155 (2008).
Forster, R. et al. Abnormal expression of the B-cell homing chemokine receptor BLR1 during the progression of acquired immunodeficiency syndrome. Blood 90, 520–525 (1997).
Acknowledgements
We thank T. W. Chun, J. Chen and A. Waldner for suggestions on the manuscript and help with the figures. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Combination antiretroviral therapy
-
Multi-drug therapy used to suppress viral replication and minimize viral resistance.
- Memory B cell
-
A quiescent antigen-experienced B cell that can rapidly differentiate into a plasma cell on re-exposure to antigen.
- Switched or unswitched antibody isotypes
-
Class switching of the immunoglobulin heavy chain from IgM and IgD to IgG or IgA or IgE allows the diversification of the antibody repertoire, generating antibody isotypes with the same antigen specificity but with different effector functions.
- Plasmablast
-
An antibody-secreting precursor of the non-dividing plasma cell that has the capacity to divide and migrate.
- Hypergammaglobulinaemia
-
Increased levels of immunoglobulin in blood serum.
- Idiopathic CD4+ T-cell lymphocytopenia
-
A disorder of unknown cause that is characterized by decreased CD4+ T-cell counts in the absence of evidence for HIV-1 or HIV-2 infection or any known immunodeficiency or therapy associated with decreased levels of CD4+ T cells.
- IgM+ memory B cell
-
An unswitched CD27+ memory B cell circulating in the peripheral blood that might be related to marginal zone B cells.
- Marginal zone B cell
-
A mature B cell that is enriched anatomically in the marginal zone of the spleen, which is the interface between red pulp and white pulp.
- Systemic lupus erythematosus
-
An autoimmune disease that is characterized by the potential for multiorgan involvement and production of antibodies that are specific for components of the cell nucleus.
- Common variable immunodeficiency
-
A primary immunodeficiency disease with a heterogeneous cause and phenotype that is characterized by hypogammaglobulinaemia.
Rights and permissions
About this article
Cite this article
Moir, S., Fauci, A. B cells in HIV infection and disease. Nat Rev Immunol 9, 235–245 (2009). https://doi.org/10.1038/nri2524
Issue Date:
DOI: https://doi.org/10.1038/nri2524