Key Points
-
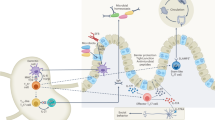
T helper 17 (TH17) cells are a newly described subset of effector T cells.
-
TH17 cells produce interleukin-17 (IL-17), IL-17F, IL-21 and IL-22.
-
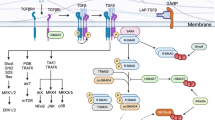
TH17-cell differentiation is initiated by transforming growth factor-β (TGFβ) and IL-6; differentiation is sustained by IL-21 and possibly completed or maintained by IL-23.
-
TH17-cell-specific genetic programming is mediated by signal transducer and activator of transcription 3 (STAT3), downstream of IL-6 and IL-21.
-
TH17 cells express two orphan nuclear receptors, retinoic-acid-receptor-related orphan receptor-α (RORα) and RORγ, which are required for TH17-cell differentiation.
Abstract
Following activation, CD4+ T cells differentiate into different lineages of helper T (TH) cells that are characterized by distinct developmental regulation and biological functions. TH17 cells have recently been identified as a new lineage of effector TH cells, and they have been shown to be important in immune responses to infectious agents, as well as in various immune diseases. Over the past two to three years, there has been a rapid progress in our understanding of the differentiation programme of TH17 cells. Here, I summarize our current knowledge of the unique gene expression, cytokine-mediated regulation and transcriptional programming of TH17 cells, and provide my personal perspectives on the future studies that are required to elucidate this lineage in more detail.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mosmann, T. R. & Coffman, R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7, 145–173 (1989).
Dong, C. & Flavell, R. A. TH1 and TH2 cells. Curr. Opin. Hematol. 8, 47–51 (2001).
Glimcher, L. H. & Murphy, K. M. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 14, 1693–1711 (2000).
Faria, A. M. & Weiner, H. L. Oral tolerance and TGF-β-producing cells. Inflamm. Allergy Drug Targets 5, 179–190 (2006).
Grazia Roncarolo, M. et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 212, 28–50 (2006).
Wing, K., Fehervari, Z. & Sakaguchi, S. Emerging possibilities in the development and function of regulatory T cells. Int. Immunol. 18, 991–1000 (2006).
Vinuesa, C. G., Tangye, S. G., Moser, B. & Mackay, C. R. Follicular B helper T cells in antibody responses and autoimmunity. Nature Rev. Immunol. 5, 853–865 (2005).
Dong, C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nature Rev. Immunol. 6, 329–334 (2006).
Harrington, L. E., Mangan, P. R. & Weaver, C. T. Expanding the effector CD4 T-cell repertoire: the TH17 lineage. Curr. Opin. Immunol. 18, 349–356 (2006).
Infante-Duarte, C., Horton, H. F., Byrne, M. C. & Kamradt, T. Microbial lipopeptides induce the production of IL-17 in TH cells. J. Immunol. 165, 6107–6115 (2000).
Dong, C. & Nurieva, R. I. Regulation of immune and autoimmune responses by ICOS. J. Autoimmun. 21, 255–260 (2003).
Murphy, C. A. et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198, 1951–1957 (2003).
Langrish, C. L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005).
Harrington, L. E. et al. Interleukin-17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunol. 6, 1123–1132 (2005).
Park, H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin-17. Nature Immunol. 6, 1133–1141 (2005).
Kolls, J. K. & Linden, A. Interleukin-17 family members and inflammation. Immunity 21, 467–476 (2004).
Moseley, T. A., Haudenschild, D. R., Rose, L. & Reddi, A. H. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 14, 155–174 (2003).
Acosta-Rodriguez, E. V. et al. Surface phenotype and antigenic specificity of human interleukin-17-producing T helper memory cells. Nature Immunol. 8, 639–646 (2007). This paper, together with references 59 and 66, describes the existence of human T H 17 cells and their possible regulation by cytokines.
Bettelli, E., Oukka, M. & Kuchroo, V. K. TH-17 cells in the circle of immunity and autoimmunity. Nature Immunol. 8, 345–350 (2007).
Steinman, L. A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell-mediated tissue damage. Nature Med. 13, 139–145 (2007).
Wei, L., Laurence, A., Elias, K. M. & O'Shea, J. J. IL-21 is produced by TH17 cells and drives IL-17 production in a STAT3-dependent manner. J. Biol. Chem. 282, 34605–34610 (2007).
Yao, Z. et al. Herpesvirus saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3, 811–821 (1995).
Toy, D. et al. Cutting edge: Interleukin-17 signals through a heteromeric receptor complex. J. Immunol. 177, 36–39 (2006).
Shalom-Barak, T., Quach, J. & Lotz, M. Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-κB. J. Biol. Chem. 273, 27467–27473 (1998).
Schwandner, R., Yamaguchi, K. & Cao, Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin-17 signal transduction. J. Exp. Med. 191, 1233–1240 (2000).
Chang, S. H., Park, H. & Dong, C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J. Biol. Chem. 281, 35603–35607 (2006).
Qian, Y. et al. The adaptor Act1 is required for interleukin-17-dependent signaling associated with autoimmune and inflammatory disease. Nature Immunol. 8, 247–256 (2007).
Ogawa, A., Andoh, A., Araki, Y., Bamba, T. & Fujiyama, Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin. Immunol. 110, 55–62 (2004).
Ruddy, M. J. et al. Functional cooperation between interleukin-17 and tumor necrosis factor-α is mediated by CCAAT/enhancer-binding protein family members. J. Biol. Chem. 279, 2559–2567 (2004).
Ye, P. et al. Requirement of interleukin-17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194, 519–527 (2001).
Nakae, S., Nambu, A., Sudo, K. & Iwakura, Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 171, 6173–6177 (2003).
Bush, K. A., Farmer, K. M., Walker, J. S. & Kirkham, B. W. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum. 46, 802–805 (2002).
Komiyama, Y. et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177, 566–573 (2006).
Hsu, H.-C. et al. Interleukin-17-producing T helper cells and interleukin-17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nature Immunol. 9, 166–175 (2008).
Nakae, S. et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17, 375–387 (2002).
Schnyder-Candrian, S. et al. Interleukin-17 is a negative regulator of established allergic asthma. J. Exp. Med. 203, 2715–2725 (2006).
Happel, K. I. et al. Cutting edge: Roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J. Immunol. 170, 4432–4436 (2003).
Shin, H. C. K., Benbernou, N., Esnault, S. & Guenounou, M. Expression of IL-17 in human memory CD45RO+ T lymphocytes and its regulation by protein kinase A pathway. Cytokine 11, 257–266 (1999).
Michel, M. L. et al. Identification of an IL-17-producing NK1.1− iNKT cell population involved in airway neutrophilia. J. Exp. Med. 204, 995–1001 (2007).
Khader, S. A. et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nature Immunol. 8, 369–377 (2007).
Shibata, K., Yamada, H., Hara, H., Kishihara, K. & Yoshikai, Y. Resident Vδ1+ γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 178, 4466–4472 (2007).
Veldhoen, M., Hocking, R. J., Atkins, C. J., Locksley, R. M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006). This paper, together with references 68 and 69, shows that T H 17-cell differentiation in mice is initiated by TGFβ and IL-6.
Hizawa, N., Kawaguchi, M., Huang, S. K. & Nishimura, M. Role of interleukin-17F in chronic inflammatory and allergic lung disease. Clin. Exp. Allergy 36, 1109–1114 (2006).
Chang, S. H. & Dong, C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 17, 435–440 (2007).
Wright, J. F. et al. Identification of an interleukin-17F/17A heterodimer in activated human CD4+ T cells. J. Biol. Chem. 282, 13447–13455 (2007).
Hurst, S. D. et al. New IL-17 family members promote TH1 or TH2 responses in the lung: in vivo function of the novel cytokine IL-25. J. Immunol. 169, 443–453 (2002).
Oda, N. et al. Interleukin-17F induces pulmonary neutrophilia and amplifies antigen-induced allergic response. Am. J. Respir. Crit. Care Med. 171, 12–18 (2005).
Kawaguchi, M. et al. Identification of a novel cytokine, ML-1, and its expression in subjects with asthma. J. Immunol. 167, 4430–4435 (2001).
Hymowitz, S. G. et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. Embo J. 20, 5332–5341 (2001).
Kuestner, R. E. et al. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J. Immunol. 179, 5462–5473 (2007).
Chung, Y. et al. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 16, 902–907 (2006).
Liang, S. C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by TH17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 (2006).
Zheng, Y. et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445, 648–651 (2007).
Wolk, K. et al. IL-22 increases the innate immunity of tissues. Immunity 21, 241–254 (2004).
Aujla, S. J. et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nature Med. 14, 275–281 (2008).
Zheng, Y. et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nature Med. 14, 282–289 (2008).
Kreymborg, K. et al. IL-22 is expressed by TH17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J. Immunol. 179, 8098–8104 (2007).
Zenewicz, L. A. et al. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 27, 647–659 (2007).
Wilson, N. J. et al. Development, cytokine profile and function of human interleukin-17-producing helper T cells. Nature Immunol. 8, 950–957 (2007).
Korn, T. et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 448, 484–487 (2007).
Nurieva, R. et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448, 480–483 (2007).
Zhou, L. et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature Immunol. 8, 967–974 (2007). References 60–62 describe the function of IL-21 in T H 17-cell differentiation.
Chtanova, T. et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-TH1/TH2 effector cells that provide help for B cells. J. Immunol. 173, 68–78 (2004).
Williams, I. R. CCR6 and CCL20: partners in intestinal immunity and lymphorganogenesis. Ann. NY Acad. Sci. 1072, 52–61 (2006).
Hirota, K. et al. Preferential recruitment of CCR6-expressing TH17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J. Exp. Med. 204, 2803–2812 (2007).
Annunziato, F. et al. Phenotypic and functional features of human TH17 cells. J. Exp. Med. 204, 1849–1861 (2007).
Nurieva, R. et al. T-cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J. 25, 2623–2633 (2006).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Mangan, P. R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006).
Coombes, J. L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Elias, K. M. et al. Retinoic acid inhibits TH17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood 111, 1013–1020 (2008).
Kang, S. G., Lim, H. W., Andrisani, O. M., Broxmeyer, H. E. & Kim, C. H. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J. Immunol. 179, 3724–3733 (2007).
Mucida, D. et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317, 256–260 (2007).
Schambach, F., Schupp, M., Lazar, M. A. & Reiner, S. L. Activation of retinoic acid receptor-α favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur. J. Immunol. 37, 2396–2399 (2007).
Sun, C.-M. et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 TReg cells via retinoic acid. J. Exp. Med. 204, 1775–1785 (2007). References 70–75 describe the function of retinoic acid in the promotion of FOXP3 expression, and references 71, 73 and 74 show that retinoic acid also inhibits T H 17-cell differentiation.
Yang, X. O. et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 282, 9358–9363 (2007). This paper, together with reference 95, describes the essential role of STAT3 in T H 17-cell differentiation.
Ivanov, I. I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006). This paper identifies RORγt as a master transcription factor for T H 17 cells.
Li, M. O., Wan, Y. Y. & Flavell, R. A. T cell-produced transforming growth factor-β1 controls T cell tolerance and regulates TH1- and TH17-cell differentiation. Immunity 26, 579–591 (2007).
Akimzhanov, A. M., Yang, X. O. & Dong, C. Chromatin remodeling of interleukin-17 (IL-17)–IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J. Biol. Chem. 282, 5969–5972 (2007).
Acosta-Rodriguez, E. V., Napolitani, G., Lanzavecchia, A. & Sallusto, F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin-17-producing human T helper cells. Nature Immunol. 8, 942–949 (2007).
Harada, M. et al. IL-21-induced Bɛ cell apoptosis mediated by natural killer T cells suppresses IgE responses. J. Exp. Med. 203, 2929–2937 (2006).
Hunter, C. A. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nature Rev. Immunol. 5, 521–531 (2005).
Cua, D. J. et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003).
Aggarwal, S., Ghilardi, N., Xie, M. H., De Sauvage, F. J. & Gurney, A. L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278, 1910–1914 (2003).
Berenson, L. S., Yang, J., Sleckman, B. P., Murphy, T. L. & Murphy, K. M. Selective requirement of p38α MAPK in cytokine-dependent, but not antigen receptor-dependent, TH1 responses. J. Immunol. 176, 4616–4621 (2006).
Thakker, P. et al. IL-23 is critical in the induction but not in the effector phase of experimental autoimmune encephalomyelitis. J. Immunol. 178, 2589–2598 (2007).
McGeachy, M. J. et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH17 cell-mediated pathology. Nature Immunol. 8, 1390–1397 (2007).
Duerr, R. H. et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314, 1461–1463 (2006).
Wellcome Trust Case Control Consortium. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nature Genet. 39, 1329–1337 (2007).
Uhlig, H. H. et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity 25, 309–318 (2006).
Okamoto, H. & Momohara, S. Interleukin-12/23 monoclonal antibody for psoriasis. N. Engl. J. Med. 356, 2003 (2007).
Langowski, J. L. et al. IL-23 promotes tumour incidence and growth. Nature 442, 461–465 (2006).
Schiffenbauer, J. et al. The induction of EAE is only partially dependent on TNF receptor signaling but requires the IL-1 type I receptor. Clin. Immunol. 95, 117–123 (2000).
Sutton, C., Brereton, C., Keogh, B., Mills, K. H. & Lavelle, E. C. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 203, 1685–1691 (2006).
Laurence, A. et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 26, 371–381 (2007).
Batten, M. et al. Interleukin-27 limits autoimmune encephalomyelitis by suppressing the development of interleukin-17-producing T cells. Nature Immunol. 7, 929–936 (2006).
Stumhofer, J. S. et al. Interleukin-27 negatively regulates the development of interleukin-17-producing T helper cells during chronic inflammation of the central nervous system. Nature Immunol. 7, 937–945 (2006).
Kleinschek, M. A. et al. IL-25 regulates TH17 function in autoimmune inflammation. J. Exp. Med. 204, 161–170 (2007).
Angkasekwinai, P. et al. Interleukin-25 promotes the initiation of proallergic type 2 responses. J. Exp. Med. 204, 1509–1517 (2007).
Afkarian, M. et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nature Immunol. 3, 549–557 (2002).
Kurata, H., Lee, H. J., O'Garra, A. & Arai, N. Ectopic expression of activated Stat6 induces the expression of TH2-specific cytokines and transcription factors in developing TH1 cells. Immunity 11, 677–688 (1999).
Mathur, A. N. et al. Stat3 and Stat4 direct development of IL-17-secreting TH cells. J. Immunol. 178, 4901–4907 (2007).
Chen, Z. et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc. Natl Acad. Sci. USA 103, 8137–8142 (2006).
Nishihara, M. et al. IL-6–gp130–STAT3 in T cells directs the development of IL-17+ TH with a minimum effect on that of TReg in the steady state. Int. Immunol. 19, 695–702 (2007).
Kimura, A., Naka, T. & Kishimoto, T. IL-6-dependent and -independent pathways in the development of interleukin-17-producing T helper cells. Proc. Natl Acad. Sci. USA 104, 12099–12104 (2007).
Yang, X. O. et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity 28, 29–39 (2008). This paper identifies RORα expression in T H 17 cells and shows that RORα and RORγt together regulate T H 17-cell development.
Harris, T. J. et al. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J. Immunol. 179, 4313–4317 (2007).
Jetten, A. M. Recent advances in the mechanisms of action and physiological functions of the retinoid-related orphan receptors (RORs). Curr. Drug Targets Inflamm. Allergy 3, 395–412 (2004).
Eberl, G. & Littman, D. R. The role of the nuclear hormone receptor RORγt in the development of lymph nodes and Peyer's patches. Immunol. Rev. 195, 81–90 (2003).
Brustle, A. et al. The development of inflammatory TH-17 cells requires interferon-regulatory factor 4. Nature Immunol. 8, 958–966 (2007).
Hu, C.-M., Jang, S. Y., Fanzo, J. C. & Pernis, A. B. Modulation of T cell cytokine production by interferon regulatory factor-4. J. Biol. Chem. 277, 49238–49246 (2002).
Lohoff, M. et al. Dysregulated T helper cell differentiation in the absence of interferon regulatory factor 4. Proc. Natl Acad. Sci. USA 99, 11808–11812 (2002).
Rengarajan, J. et al. Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J. Exp. Med. 195, 1003–1012 (2002).
Lee, G. R., Kim, S. T., Spilianakis, C. G., Fields, P. E. & Flavell, R. A. T helper cell differentiation: regulation by cis elements and epigenetics. Immunity 24, 369–379 (2006).
Moisan, J., Grenningloh, R., Bettelli, E., Oukka, M. & Ho, I. C. Ets-1 is a negative regulator of TH17 differentiation. J. Exp. Med. 204, 2825–2835 (2007).
Young, D. A. et al. Blockade of the interleukin-21/interleukin-21 receptor pathway ameliorates disease in animal models of rheumatoid arthritis. Arthritis Rheum. 56, 1152–1163 (2007).
Spolski, R. & Leonard, W. J. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu. Rev. Immunol. 26, 57–79 (2008).
Herber, D. et al. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J. Immunol. 178, 3822–3830 (2007).
Acknowledgements
I thank my past and current colleagues in my group and our many collaborators for their scientific contributions to the knowledge described in this Review. My research is funded by the National Institutes of Health (USA), the University of Texas MD Anderson Cancer Center, the Cancer Research Institute and the American Lung Association.
Author information
Authors and Affiliations
Related links
Glossary
- T helper 3 (TH3) cells
-
A regulatory T-cell subset that was originally thought to be involved in oral tolerance and that mainly secretes TGFβ. TGFβ produced by TH3 cells provides help for IgA class switching and has suppressive effects on both TH1 and TH2 cells. As the expression of CD25 and FOXP3 is induced in T cells under experimental TH3-cell differentiation conditions (T-cell receptor stimulation in the presence of IL-4, IL-10 and TGFβ), TH3 cells should be referred to as inducible regulatory T cells that proliferate in the periphery.
- T regulatory type 1 (TR1) cells
-
A subset of CD4+ regulatory T cells that secrete high levels of IL-10 and that downregulate TH1- and TH2-cell responses in vitro and in vivo by a contact-independent mechanism(s) mediated by the secretion of soluble IL10 and TGFβ1.
- Microarray analysis
-
A technique for measuring the transcription of genes. It involves hybridization of fluorescently labelled cDNA prepared from a cell or tissue of interest with glass slides or other surfaces dotted with thousands of oligonucleotides or cDNA, ideally representing all expressed genes in the species.
- Experimental autoimmune encephalomyelitis
-
(EAE). An animal model of multiple sclerosis. EAE can be induced in several mammalian species by immunization with myelin-derived antigens together with adjuvant. The immunized animals develop a paralytic disease that has several pathological features in common with multiple sclerosis in humans.
- Systemic lupus erythematosus
-
(SLE). An autoimmune disease in which autoantibodies that are specific for DNA, RNA or proteins associated with nucleic acids form immune complexes that damage small blood vessels, particularly in the kidney. Patients with SLE generally have abnormal B- and T-cell function.
- Germinal centre
-
A highly specialized and dynamic microenvironment that gives rise to secondary B-cell follicles during an immune response. It is the main site of B-cell maturation, leading to the generation of memory B cells and plasma cells that produce high-affinity antibodies.
- Natural killer T (NKT) cell
-
A T cell that expresses both NK-cell receptors and an αβ-TCR. Some mouse NKT cells express an invariant TCR that uses the Vα14 variable region of the TCR α-chain and recognizes CD1d-associated antigen. NKT cells are characterized by cytolytic activity and the rapid production of cytokines, including IFNγ and IL-4, and they might regulate the function of other T cells.
- γδ T cell
-
A T cell that expresses a T-cell receptor consisting of a γ-chain and a δ-chain. These T cells are present mainly in the intestinal epithelium as intraepithelial lymphocytes (IELs). Although the exact function of γδ T cells (or IELs) is still unknown, it has been proposed that mucosal γδ T cells are involved in innate immune responses by the mucosal immune system.
- Common cytokine receptor γ-chain
-
(γc). A type I cytokine receptor chain that is shared by the receptors for IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21.
- Epigenetic
-
This term refers to the heritable, but potentially reversible, states of gene activity that are imposed by the structure of chromatin or covalent modifications of DNA and histones.
Rights and permissions
About this article
Cite this article
Dong, C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol 8, 337–348 (2008). https://doi.org/10.1038/nri2295
Issue Date:
DOI: https://doi.org/10.1038/nri2295