Key Points
-
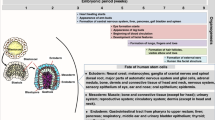
The fetus is an allograft onto the mother and is at risk of rejection, so there is a strong T helper 2 (TH2)-cell bias to fetal innate immune responses, a tendency also initially manifest in the newborn. The production of pro-inflammatory/TH1-cell-polarizing cytokines such as tumour-necrosis factor (TNF), interferon-γ (IFNγ), and interleukin-12 (IL-12) is impaired.
-
Neonatal monocytes and antigen-presenting cells (APCs) show reduced production of TNF, IL-12 and IFNγ, but preserved production of IL-6, IL-10 and IL-23. Mechanisms for this polarization include elevated cytosolic concentrations of the second messenger cyclic AMP (cAMP), reduced myeloid differentiation primary-response gene 88 (MyD88) expression and defects in nucleosome remodelling.
-
Birth initiates an acute-phase response characterized by rising serum levels of IL-6 and increases in IL-6-inducible hepatocyte products such as C-reactive protein (CRP) and lipopolysaccharide (LPS)-binding protein. It is speculated that this response might serve to clear microbes and/or microbial products that might translocate across mucosal barriers during birth and/or initial colonization.
-
Shortly after birth, upon initial microbial colonization, the neonatal intestinal tract downregulates its inflammatory responses to endotoxin to avoid over-exuberant and potentially harmful reactions to common Gram-negative bacterial flora.
-
The neonatal respiratory tract manifests age-dependent maturation of Toll-like receptor (TLR) responses and expression of antimicrobial proteins and peptides (APPs). As a major portal for aeroallergens and aeroadjuvants (for example, microbial products that are TLR agonists), the respiratory tract might mediate maturation of neonatal and infant TH1-cell-type responses by repeated low-dose exposure to environmental TLR agonists, as posited by the hygiene hypothesis.
-
Neonatal neutrophils show qualitative defects in reduced integrin and selectin expression, reduced expression of some antimicrobial proteins and impaired priming of the phagocyte oxidase. In addition, neonates experience a quantitative neutrophil deficiency during stress conditions.
-
Robust responses to certain microbial stimuli, including viral single-stranded RNAs and related imidazoquinoline compounds that activate cells through TLR8, represent exceptions to the generally impaired production of pro-inflammatory/TH1-cell-polarizing cytokines.
-
On-going efforts seek to leverage our newfound knowledge of innate immunity to diagnose, prevent and treat neonatal diseases. Such approaches might include measuring components of innate immunity to diagnose infection, using APPs as anti-infective agents or using TLR agonists as vaccine adjuvants.
Abstract
The fetus and newborn face a complex set of immunological demands, including protection against infection, avoidance of harmful inflammatory immune responses that can lead to pre-term delivery, and balancing the transition from a sterile intra-uterine environment to a world that is rich in foreign antigens. These demands shape a distinct neonatal innate immune system that is biased against the production of pro-inflammatory cytokines. This bias renders newborns at risk of infection and impairs responses to many vaccines. This Review describes innate immunity in newborns and discusses how this knowledge might be used to prevent and treat infection in this vulnerable population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Klein, J. & Remington, J. in Infectious Diseases of the Fetus and Newborn Infant (eds Remington, J. & Klein, J.) 1–23 (W. B. Saunders Company, Philadelphia, 2001).
McDonagh, S. et al. Viral and bacterial pathogens at the maternal–fetal interface. J. Infect. Dis. 190, 826–834 (2004).
Makhseed, M. et al. Th1 and Th2 cytokine profiles in recurrent aborters with successful pregnancy and with subsequent abortions. Hum. Reprod. 16, 2219–2226 (2001).
Marchini, G. et al. Erythema toxicum neonatorum is an innate immune response to commensal microbes penetrated into the skin of the newborn infant. Pediatr. Res. 58, 613–616 (2005). This paper reveals that erythema toxicum lesions contain common Gram-positive bacteria (coagulase-negative staphylococci) that penetrate the skin through hair follicles to recruit APCs, correlating with increases in body temperature that are consistent with an acute-phase response at birth.
Karlsson, H., Hessle, C. & Rudin, A. Innate immune responses of human neonatal cells to bacteria from the normal gastrointestinal flora. Infect. Immun. 70, 6688–6696 (2002).
Adkins, B., Leclerc, C. & Marshall-Clarke, S. Neonatal adaptive immunity comes of age. Nature Rev. Immunol. 4, 553–564 (2004).
Krishnan, S., Craven, M., Welliver, R. C., Ahmad, N. & Halonen, M. Differences in participation of innate and adaptive immunity to respiratory syncytial virus in adults and neonates. J. Infect. Disease 188, 433–439 (2003).
Firth, M. A., Shewen, P. E. & Hodgins, D. C. Passive and active components of neonatal innate immune defenses. Anim. Health Res. Rev. 6, 143–158 (2005).
Janeway, C. A., Jr. & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002).
Vitoratos, N. et al. Elevated circulating IL-1β and TNF-α, and unaltered IL-6 in first-trimester pregnancies complicated by threatened abortion with an adverse outcome. Mediators Inflamm. 2006, 1–6 (2006).
Marodi, L. Innate cellular immune responses in newborns. Clin. Immunol. 118, 137–144 (2006).
Angelone, D. et al. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-α production in vitro and in vivo. Pediatr. Res. 60, 205–209 (2006). This paper shows that in vitro TLR-mediated responses by monocytes from human newborns, as well as basal ( in vivo ) neonatal plasma, are characterized by low levels of TNF but high levels of IL-6.
Vanden Eijnden, S., Goriely, S., De Wit, D., Goldman, M. & Willems, F. Preferential production of the IL-12(p40)/IL-23(p19) heterodimer by dendritic cells from human newborns. Eur. J. Immunol. 36, 21–26 (2006).
Chelvarajan, R. L. et al. Defective macrophage function in neonates and its impact on unresponsiveness of neonates to polysaccharide antigens. J. Leukoc. Biol. 75, 982–994 (2004).
Siegrist, C. A. Vaccination in the neonatal period and early infancy. Int. Rev. Immunol. 19, 195–219 (2000).
Ng, N., Lam, D., Paulus, P., Batzer, G. & Horner, A. A. House dust extracts have both TH2 adjuvant and tolerogenic activities. J. Allergy Clin. Immunol. 117, 1074–1081 (2006).
Bach, J. F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 347, 911–920 (2002).
Liu, A. H. & Leung, D. Y. Renaissance of the hygiene hypothesis. J. Allergy Clin. Immunol. 117, 1063–1066 (2006).
Imler, J. L. & Hoffmann, J. A. Toll receptors in innate immunity. Trends Cell Biol. 11, 304–311 (2001).
Doyle, S. L. & O'Neill L. A. Toll-like receptors: From the discovery of NFκB to new insights into transcriptional regulations in innate immunity. Biochem. Pharmacol. 72, 1102–1113 (2006).
Akira, S. Mammalian Toll-like receptors. Curr. Opin. Immunol. 15, 5–11 (2003).
Ganz, T. Antimicrobial polypeptides. J. Leukoc. Biol. 75, 34–38 (2004).
Levy, O. Antimicrobial proteins and peptides: anti-infective molecules of mammalian leukocytes. J. Leukoc. Biol. 76, 909–925 (2004).
Fasciano, S. & Li, L. Intervention of Toll-like receptor-mediated human innate immunity and inflammation by synthetic compounds and naturally occurring products. Curr. Med. Chem. 13, 1389–1395 (2006).
Larson, A. A. & Dinulos, J. G. Cutaneous bacterial infections in the newborn. Curr. Opin. Pediatr. 17, 481–485 (2005).
Tollin, M. et al. Vernix caseosa as a multi-component defence system based on polypeptides, lipids and their interactions. Cell. Mol. Life Sci. 62, 2390–2399 (2005).
Zasloff, M. Vernix, the newborn, and innate defense. Pediatr. Res. 53, 203–204 (2003).
Yoshio, H. et al. Antimicrobial polypeptides of human vernix caseosa and amniotic fluid: implications for newborn innate defense. Pediatr. Res. 53, 211–216 (2003).
Tollin, M., Jagerbrink, T., Haraldsson, A., Agerberth, B. & Jornvall, H. Proteome analysis of vernix caseosa. Pediatr. Res. 60, 430–434 (2006).
Dorschner, R. A., Lin, K. H., Murakami, M. & Gallo, R. L. Neonatal skin in mice and humans expresses increased levels of antimicrobial peptides: innate immunity during development of the adaptive response. Pediatr. Res. 53, 566–572 (2003). Shows that at birth, neonatal skin epithelia express increased levels of the antimicrobial peptides β-defensin and cathelicidin.
Marchini, G., Berggren, V., Djilali-Merzoug, R. & Hansson, L. O. The birth process initiates an acute phase reaction in the fetus–newborn infant. Acta Paediatr. 89, 1082–1086 (2000).
Angelone, D. F. et al. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-α production in vitro and in vivo. Pediatr. Res. 60, 205–209 (2006).
Abreu, M. T., Fukata, M. & Arditi, M. TLR signaling in the gut in health and disease. J. Immunol. 174, 4453–4460 (2005).
Fusunyan, R. D., Nanthakumar, N. N., Baldeon, M. E. & Walker, W. A. Evidence for an innate immune response in the immature human intestine: toll-like receptors on fetal enterocytes. Pediatr. Res. 49, 589–593 (2001).
Gioannini, T. L. et al. Isolation of an endotoxin–MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc. Natl Acad. Sci. USA 101, 4186–4191 (2004).
Lotz, M. et al. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J. Exp. Med. 203, 973–984 (2006). A landmark study revealing a mechanism by which initial intestinal endotoxin exposure at birth downregulates subsequent responses, paving the way for commensal interactions.
Nanthakumar, N. N., Fusunyan, R. D., Sanderson, I. & Walker, W. A. Inflammation in the developing human intestine: a possible pathophysiologic contribution to necrotizing enterocolitis. Proc. Natl Acad. Sci. USA 97, 6043–6048 (2000).
Fanaro, S., Chierici, R., Guerrini, P. & Vigi, V. Intestinal microflora in early infancy: composition and development. Acta Paediatr. 91 (Suppl.), 48–55 (2003).
Rakoff-Nahoum, S. & Medzhitov, R. Role of the innate immune system and host-commensal mutualism. Curr. Top. Microbiol. Immunol. 308, 1–18 (2006).
Sherman, M. P., Bennett, S. H., Hwang, F. F., Sherman, J. & Bevins, C. L. Paneth cells and antibacterial host defense in neonatal small intestine. Infect. Immun. 73, 6143–6146 (2005).
Jilling, T. et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J. Immunol. 177, 3273–3282 (2006).
Lin, P. W. & Stoll, B. J. Necrotising enterocolitis. Lancet 368, 1271–1283 (2006).
Newburg, D. S. & Walker, W. A. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr. Res. 61, 2–8 (2007).
Labbok, M. H., Clark, D. & Goldman, A. S. Breastfeeding: maintaining an irreplaceable immunological resource. Nature Rev. Immunol. 4, 565–572 (2004).
Armogida, S. A., Yannaras, N. M., Melton, A. L. & Srivastava, M. D. Identification and quantification of innate immune system mediators in human breast milk. Allergy Asthma Proc. 25, 297–304 (2004).
LeBouder, E. et al. Soluble forms of Toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk. J. Immunol. 171, 6680–6689 (2003).
LeBouder, E. et al. Modulation of neonatal microbial recognition: TLR-mediated innate immune responses are specifically and differentially modulated by human milk. J. Immunol. 176, 3742–3752 (2006).
Jones, C. A. et al. Reduced soluble CD14 levels in amniotic fluid and breast milk are associated with the subsequent development of atopy, eczema, or both. J. Allergy Clin. Immunol. 109, 858–866 (2002).
Harju, K., Glumoff, V. & Hallman, M. Ontogeny of Toll-like receptors Tlr2 and Tlr4 in mice. Pediatr. Res. 49, 81–83 (2001).
Martin, T. R., Ruzinski, J. T., Wilson, C. B. & Skerrett, S. J. Effects of endotoxin in the lungs of neonatal rats: age-dependent impairment of the inflammatory response. J. Infect. Dis. 171, 134–144 (1995).
Liu, L., Roberts, A. A. & Ganz, T. By IL-1 signaling, monocyte-derived cells dramatically enhance the epidermal antimicrobial response to lipopolysaccharide. J. Immunol. 170, 575–580 (2003).
Starner, T. D., Agerberth, B., Gudmundsson, G. H. & McCray, P. B., Jr. Expression and activity of β-defensins and LL-37 in the developing human lung. J. Immunol. 174, 1608–1615 (2005).
Elahi, S. et al. The host defense peptide β-defensin 1 confers protection against Bordetella pertussis in newborn piglets. Infect. Immun. 74, 2338–2352 (2006). Impressive correlation of β-defensin expression in neonatal respiratory epithelium and resistance to B. pertussis infection.
Nogueira-Silva, C., Santos, M., Baptista, M. J., Moura, R. S. & Correia-Pinto, J. IL-6 is constitutively expressed during lung morphogenesis and enhances fetal lung explant branching. Pediatr. Res. 60, 530–536 (2006).
Schelonka, R. L., Katz, B., Waites, K. B. & Benjamin, D. K., Jr. Critical appraisal of the role of Ureaplasma in the development of bronchopulmonary dysplasia with metaanalytic techniques. Pediatr. Infect. Dis. J. 24, 1033–1039 (2005).
Peltier, M. R., Freeman, A. J., Mu, H. H. & Cole, B. C. Characterization of the macrophage-stimulating activity from Ureaplasma urealyticum. Am. J. Reprod. Immunol. 57, 186–192 (2007).
Manimtim, W. M. et al. Ureaplasma urealyticum modulates endotoxin-induced cytokine release by human monocytes derived from preterm and term newborns and adults. Infect. Immun. 69, 3906–3915 (2001).
Prince, L. S., Dieperink, H. I., Okoh, V. O., Fierro-Perez, G. A. & Lallone, R. L. Toll-like receptor signaling inhibits structural development of the distal fetal mouse lung. Dev. Dyn. 233, 553–561 (2005).
Benjamin, J. T. et al. FGF-10 is decreased in bronchopulmonary dysplasia and suppressed by Toll-like receptor activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L550–L558 (2007).
Tulic, M. K. et al. Role of toll-like receptor 4 in protection by bacterial lipopolysaccharide in the nasal mucosa of atopic children but not adults. Lancet 363, 1689–1697 (2004).
van Strien, R. T. et al. Microbial exposure of rural school children, as assessed by levels of N-acetyl-muramic acid in mattress dust, and its association with respiratory health. J. Allergy Clin. Immunol. 113, 860–867 (2004).
Roponen, M., Hyvarinen, A., Hirvonen, M. R., Keski-Nisula, L. & Pekkanen, J. Change in IFN-γ-producing capacity in early life and exposure to environmental microbes. J. Allergy Clin. Immunol. 116, 1048–1052 (2005).
Vercelli, D. Mechanisms of the hygiene hypothesis — molecular and otherwise. Curr. Opin. Immunol. 18, 733–737 (2006).
Godfrey, W. R. et al. Cord blood CD4+CD25+-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood 105, 750–758 (2005).
Pasare, C. & Medzhitov, R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science 299, 1033–1036 (2003).
Peng, G. et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science 309, 1380–1384 (2005).
Levy, O. et al. Enhancement of neonatal innate defense: effects of adding an N-terminal recombinant fragment of bactericidal/permeability-increasing protein (rBPI21) on growth and TNF-inducing activity of Gram-negative bacteria tested in neonatal cord blood ex vivo. Infect. Immun. 68, 5120–5125 (2000).
Carroll, M. C. The complement system in B cell regulation. Mol Immunol 41, 141–146 (2004).
Pihlgren, M. et al. Influence of complement C3 amount on IgG responses in early life: immunization with C3b-conjugated antigen increases murine neonatal antibody responses. Vaccine 23, 329–335 (2004).
Jokic, M. et al. Fetal distress increases interleukin-6 and interleukin-8 and decreases tumour necrosis factor-α cord blood levels in noninfected full-term neonates. Br. J. Ob. Gyn. 107, 420–425 (2000).
Levy, O. et al. The adenosine system selectively inhibits TLR-mediated TNF-α production in the human newborn. J. Immunol. 177, 1956–1966 (2006). Shows, in human neonatal cord blood, that high plasma adenosine levels and increased mononuclear-cell sensitivity to adenosine action results in greatly increased cellular cAMP concentrations that impair TLR2-mediated TNF production but preserve production of IL-6.
Hasko, G. & Cronstein, B. N. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 25, 33–39 (2004).
Marchini, G. et al. Erythema toxicum neonatorum: an immunohistochemical analysis. Pediatr. Dermatol. 18, 177–187 (2001).
Aittoniemi, J. et al. Age-dependent variation in the serum concentration of mannan-binding protein. Acta Paediatr. 85, 906–909 (1996).
Levy, O. et al. Selective impairment of Toll-like receptor-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-α induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod but preserves response to R-848. J. Immunol. 173, 4627–4634 (2004).
Burgess-Beusse, B. L. & Darlington, G. J. C/EBPα is critical for the neonatal acute-phase response to inflammation. Mol. Cell Biol. 18, 7269–7277 (1998).
Gangneux, C. et al. The inflammation-induced down-regulation of plasma Fetuin-A (α2HS-Glycoprotein) in liver results from the loss of interaction between long C/EBP isoforms at two neighbouring binding sites. Nucleic Acids Res. 31, 5957–5970 (2003).
Dziegielewska, K. M., Andersen, N. A. & Saunders, N. R. Modification of macrophage response to lipopolysaccharide by fetuin. Immunol. Lett. 60, 31–35 (1998).
Dziegielewska, K. M. & Andersen, N. A. The fetal glycoprotein, fetuin, counteracts ill-effects of the bacterial endotoxin, lipopolysaccharide, in pregnancy. Biol. Neonate 74, 372–375 (1998).
Ombrellino, M. et al. Fetuin, a negative acute phase protein, attenuates TNF synthesis and the innate inflammatory response to carrageenan. Shock 15, 181–185 (2001).
Kitchens, R. L. & Thompson, P. A. Modulatory effects of sCD14 and LBP on LPS–host cell interactions. J. Endotoxin Res. 11, 225–229 (2005).
Carr, R. Neutrophil production and function in newborn infants. Br. J. Haematol. 110, 18–28 (2000).
Urlichs, F. & Speer, C. P. Neutrophil function in preterm and term infants. Neonatology Rev. 5, e417–e430 (2004).
Henneke, P. & Berner, R. Interaction of neonatal phagocytes with group B streptococcus: recognition and response. Infect. Immun. 74, 3085–3095 (2006).
Reddy, R. K., Xia, Y., Hanikýrová, M. & Ross, G. D. A mixed population of immature and mature leucocytes in umbilical cord blood results in a reduced expression and function of CR3 (CD11b/CD18). Clin. Exper. Immunol. 114, 462–467 (1998).
Rebuck, N., Gibson, A. & Finn, A. Neutrophil adhesion molecules in term and premature infants: normal or enhanced leucocyte integrins but defective L-selectin expression and shedding. Clin. Exper. Immunol. 101, 183–189 (1995).
Schultz, C. et al. Enhanced interleukin-6 and interleukin-8 synthesis in term and preterm infants. Pediatr. Res. 51, 317–322 (2002).
Jones, S. A. Directing transition from innate to acquired immunity: defining a role for IL-6. J. Immunol. 175, 3463–3468 (2005).
Ambruso, D. R., Bentwood, B., Henson, P. M. & Johnston, R. B., Jr. Oxidative metabolism of cord blood neutrophils: relationship to content and degranulation of cytoplasmic granules. Pediatr. Res. 18, 1148–1153 (1984).
Levy, O. et al. Impaired innate immunity in the newborn: newborn neutrophils are deficient in bactericidal/permeability-increasing protein (BPI). Pediatrics 104, 1327–1333 (1999).
Bjorkqvist, M., Jurstrand, M., Bodin, L., Fredlund, H. & Schollin, J. Defective neutrophil oxidative burst in preterm newborns on exposure to coagulase-negative staphylococci. Pediatr. Res. 55, 966–971 (2004).
Qing, G., Rajaraman, K. & Bortolussi, R. Diminished priming of neonatal polymorphonuclear leukocytes by lipopolysaccharide is associated with reduced CD14 expression. Infect. Immun. 63, 248–252 (1995).
Yan, S. R., Byers, D. M. & Bortolussi, R. Role of protein tyrosine kinase p53/56lyn in diminished lipopolysaccharide priming of formylmethionylleucyl — phenylalanine-induced superoxide production in human newborn neutrophils. Infect. Immun. 72, 6455–6462 (2004).
Jones, C. A., Holloway, J. A. & Warner, J. O. Phenotype of fetal monocytes and B lymphocytes during the third trimester of pregnancy. J. Reprod. Immunol. 56, 45–60 (2002).
Dakic, A. et al. Development of the dendritic cell system during mouse ontogeny. J. Immunol. 172, 1018–1027 (2004).
Sun, C. M., Fiette, L., Tanguy, M., Leclerc, C. & Lo-Man, R. Ontogeny and innate properties of neonatal dendritic cells. Blood 102, 585–591 (2003).
Sun, C. M., Deriaud, E., Leclerc, C. & Lo-Man, R. Upon TLR9 signaling, CD5+ B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity 22, 467–477 (2005).
Hunt, D. W., Huppertz, H. I., Jiang, H. J. & Petty, R. E. Studies of human cord blood dendritic cells: evidence for functional immaturity. Blood 84, 4333–4343 (1994).
Darmochwal-Kolarz, D. et al. CD1c+ immature myeloid dendritic cells are predominant in cord blood of healthy neonates. Immunol. Lett. 91, 71–74 (2004).
Levy, O., Suter, E. E., Miller, R. L. & Wessels, M. R. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood 108, 1284–1290 (2006).
Peltier, M. R., Freeman, A. J., Mu, H. H. & Cole, B. C. Characterization and partial purification of a macrophage-stimulating factor from Mycoplasma hominis. Am. J. Reprod. Immunol. 54, 342–351 (2005).
van der Graaf, C. A., Netea, M. G., Verschueren, I., van der Meer, J. W. & Kullberg, B. J. Differential cytokine production and Toll-like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Infect. Immun. 73, 7458–7464 (2005).
Kurt-Jones, E. A. et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nature Immunol. 1, 398–401 (2000).
Yarovinsky, F. et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308, 1626–1629 (2005).
Forster-Waldl, E. et al. Monocyte toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr. Res. 58, 121–124 (2005).
Yan, S. R. et al. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect. Immun. 72, 1223–1229 (2004).
Cohen, L. et al. CD14-independent responses to LPS require a serum factor that is absent from neonates. J. Immunol. 155, 5337–5342 (1995).
De Wit, D. et al. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood 103, 1030–1032 (2004).
Aksoy, E. et al. Interferon regulatory factor 3-dependent responses to lipopolysaccharide are selectively blunted in cord blood cells. Blood 30 November 2006 (doi:10.1182/blood-2006-06-027862).
Prescott, S. L. et al. Cytosine-phosphate-guanine motifs fail to promote T-helper type 1-polarized responses in human neonatal mononuclear cells. Clin. Exp. Allergy 35, 358–366 (2005).
Wong, O. H., Huang, F. P. & Chiang, A. K. Differential responses of cord and adult blood-derived dendritic cells to dying cells. Immunology 116, 13–20 (2005).
Mandron, M. et al. Human dendritic cells conditioned with Staphylococcus aureus enterotoxin B promote TH2 cell polarization. J. Allergy Clin. Immunol. 117, 1141–1147 (2006).
Cusumano, V. et al. Neonatal hypersusceptibility to endotoxin correlates with increased tumor necrosis factor production in mice. J. Infect. Disease 176, 168–176 (1997).
Kao, C. Y. et al. IL-17 markedly up-regulates β-defensin-2 expression in human airway epithelium via JAK and NF-κB signaling pathways. J. Immunol. 173, 3482–3491 (2004).
Kurt-Jones, E. A. et al. The role of toll-like receptors in herpes simplex infection in neonates. J. Infect. Dis. 191, 746–748 (2005).
Wynn, J. et al. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock (in the press).
Xu, D. X. et al. Tumor necrosis factor a partially contributes to lipopolysaccharide-induced intra-uterine fetal growth restriction and skeletal development retardation in mice. Toxicol. Lett. 163, 20–29 (2006).
Lehnardt, S. et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl Acad. Sci. USA 100, 8514–8519 (2003).
Sherwin, C. & Fern, R. Acute lipopolysaccharide-mediated injury in neonatal white matter glia: role of TNF-α, IL-1β, and calcium. J. Immunol. 175, 155–161 (2005).
Lewis, D. B. & Wilson, C. B. in Infectious Diseases of the Fetus and Newborn Infant (eds Remington, J. & Klein, J.) 25–138 (W. B. Saunders, Philadelphia, 2001).
Zhang, J., Bui, T. N., Xiang, J. & Lin, A. Cyclic AMP inhibits p38 activation via CREB-induced dynein light chain. Mol. Cell. Biol. 26, 1223–1234 (2006).
Ajizian, S. J., English, B. K. & Meals, E. A. Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-γ. J. Infect. Disease 179, 939–944 (1999).
Levy, O. et al. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-α induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J. Immunol. 173, 4627–4634 (2004).
Sadeghi, K. et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J. Infect. Dis. 195, 296–302 (2007).
Schnurr, M. et al. Extracellular nucleotide signaling by P2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: a novel role for the cAMP pathway. Blood 105, 1582–1589 (2005).
Goriely, S. et al. A defect in nucleosome remodeling prevents IL-12(p35) gene transcription in neonatal dendritic cells. J. Exp. Med. 199, 1011–1016 (2004). Shows that expression of IL-12 p35 by human neonatal DCs is regulated at the chromatin level by a defect in nucleosome remodelling.
Krieg, A. M. Therapeutic potential of Toll-like receptor 9 activation. Nature Rev. Drug Discov. 5, 471–484 (2006).
Pedras-Vasconcelos, J. A. et al. CpG oligodeoxynucleotides protect newborn mice from a lethal challenge with the neurotropic Tacaribe arenavirus. J. Immunol. 176, 4940–4949 (2006).
Levy, O. et al. Critical role of the complement system in Group B streptococcus-induced tumor necrosis factor alpha release. Infect. Immun. 71, 6344–6353 (2003).
Vekemans, J. et al. Neonatal bacillus Calmette–Guérin vaccination induces adult-like IFN-γ production by CD4+ T lymphocytes. Eur. J. Immunol. 31, 1531–1535 (2001).
Strunk, T. & Burgner, D. Genetic susceptibility to neonatal infection. Curr. Opin. Infect. Dis. 19, 259–263 (2006).
Mishra, U. K., Jacobs, S. E., Doyle, L. W. & Garland, S. M. Newer approaches to the diagnosis of early onset neonatal sepsis. Arch. Dis. Child. Fetal Neonatal Ed. 91, F208–F212 (2006).
Viemann, D. et al. Expression of toll-like receptors in neonatal sepsis. Pediatr. Res. 58, 654–659 (2005).
Carr, R., Modi, N. & Dore, C. G-CSF and GM-CSF for treating or preventing neonatal infections. Cochrane Database Syst. Rev., 3, CD003066 (2003).
Ito, S. et al. CpG oligodeoxynucleotides enhance neonatal resistance to Listeria infection. J. Immunol. 174, 777–782 (2005). Shows that administration of CpG ODNs can protect newborn mice from subsequent challenge with L. monocytogenes.
Barrier, M. et al. Oral and intraperitoneal administration of phosphorothioate oligodeoxynucleotides leads to control of Cryptosporidium parvum infection in neonatal mice. J. Infect. Dis. 193, 1400–1407 (2006).
Lambert, P. H. Vaccines for the world: major challenges for the future. Southeast Asian J. Trop. Med. Public Health 28, 122–126 (1997).
Kovarik, J. et al. CpG oligodeoxynucleotides can circumvent the Th2 polarization of neonatal responses to vaccines but may fail to fully redirect Th2 responses established by neonatal priming. J. Immunol. 162, 1611–1617 (1999).
Hein, M., Valore, E. V., Helmig, R. B., Uldbjerg, N. & Ganz, T. Antimicrobial factors in the cervical mucus plug. Am. J. Obstet. Gynecol. 187, 137–144 (2002).
Schaefer, T. M., Desouza, K., Fahey, J. V., Beagley, K. W. & Wira, C. R. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology 112, 428–436 (2004).
Schaefer, T. M., Fahey, J. V., Wright, J. A. & Wira, C. R. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C). J. Immunol. 174, 992–1002 (2005).
Abrahams, V. M. et al. Divergent trophoblast responses to bacterial products mediated by TLRs. J. Immunol. 173, 4286–4296 (2004).
Huppertz, B., Kadyrov, M. & Kingdom, J. C. Apoptosis and its role in the trophoblast. Am. J. Obstet. Gynecol. 195, 29–39 (2006).
Beijar, E. C., Mallard, C. & Powell, T. L. Expression and subcellular localization of TLR-4 in term and first trimester human placenta. Placenta 27, 322–326 (2006).
Espinoza, J. et al. Lipopolysaccharide-binding protein in microbial invasion of the amniotic cavity and human parturition. J. Matern. Fetal Med. 12, 313–321 (2002).
Kim, H. S. et al. Endotoxin-neutralizing antimicrobial proteins of the human placenta. J. Immunol. 168, 2356–2364 (2002).
Pacora, P. et al. Lactoferrin in intrauterine infection, human parturition, and rupture of fetal membranes. Am. J. Obstet. Gyn. 183, 904–910 (2000).
Cherry, S. H., Filler, M. & Harvey, H. Lysozyme content of amniotic fluid. Am. J. Obstet. Gyn. 116, 639–642 (1973).
Koyama, M. et al. Elevations of group II phospholipase A2 concentrations in serum and amniotic fluid in association with preterm labor. Am. J. Obstet. Gyn. 183, 1537–1543 (2000).
Weinrauch, Y., Abad, C., Liang, N. S., Lowry, S. F. & Weiss, J. Mobilization of potent plasma bactericidal activity during systemic bacterial challenge. Role of group IIA phospholipase A2. J. Clin. Invest. 102, 633–638 (1998).
Mathai, M., Jairaj, P., Thangavelu, C. P., Mathai, E. & Balasubramaniam, N. Antimicrobial activity of amniotic fluid in South Indian women. Br. J. Obstet. Gynaecol. 91, 560–564 (1984).
Durr, M. & Peschel, A. Chemokines meet defensins: the merging concepts of chemoattractants and antimicrobial peptides in host defense. Infect. Immun. 70, 6515–6517 (2002).
Bowdish, D. M., Davidson, D. J. & Hancock, R. E. Immunomodulatory properties of defensins and cathelicidins. Curr. Top. Microbiol. Immunol. 306, 27–66 (2006).
Dominguez, F., Pellicer, A. & Simon, C. The chemokine connection: hormonal and embryonic regulation at the human maternal-embryonic interface — a review. Placenta 24, S48–S55 (2003).
Liu, C. et al. The role of CCL21 in recruitment of T-precursor cells to fetal thymi. Blood 105, 31–39 (2005).
Meurens, F. et al. Expression of mucosal chemokines TECK/CCL25 and MEC/CCL28 during fetal development of the ovine mucosal immune system. Immunol. 120, 544–555 (2007).
Plotkin, J., Prockop, S. E., Lepique, A. & Petrie, H. T. Critical role for CXCR4 signaling in progenitor localization and T cell differentiation in the postnatal thymus. J. Immunol. 171, 4521–4527 (2003).
Garvy, B. A. & Qureshi, M. H. Delayed inflammatory response to Pneumocystis carinii infection in neonatal mice is due to an inadequate lung environment. J. Immunol. 165, 6480–6486 (2000).
Krolak-Olejnik, B., Beck, B. & Olejnik, I. Umbilical serum concentrations of chemokines (RANTES and MGSA/GRO-α) in preterm and term neonates. Pediatr. Int. 48, 586–590 (2006).
Zhou, J. et al. Differential expression of chemokines and their receptors in adult and neonatal macrophages infected with human or avian influenza viruses. J. Infect. Dis. 194, 61–70 (2006).
Meddows-Taylor, S. et al. Reduced ability of newborns to produce CCL3 is associated with increased susceptibility to perinatal human immunodeficiency virus 1 transmission. J. Gen. Virol. 87, 2055–2065 (2006).
Ng, P. C. et al. IP-10 is an early diagnostic marker for identification of late-onset bacterial infection in preterm infants. Pediatr. Res. 61, 93–98 (2007).
Collado-Hidalgo, A., Sung, C. & Cole, S. Adrenergic inhibition of innate anti-viral response: PKA blockade of Type I interferon gene transcription mediates catecholamine support for HIV-1 replication. Brain Behav. Immun. 20, 552–563 (2006).
Peters, A. M., Bertram, P., Gahr, M. & Speer, C. P. Reduced secretion of interleukin-1 and tumor necrosis factor-α by neonatal monocytes. Biol. Neonate 63, 157–162 (1993).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Bowen, R. S., Gu, Y., Zhang, Y., Lewis, D. F. & Wang, Y. Hypoxia promotes interleukin-6 and -8 but reduces interleukin-10 production by placental trophoblast cells from preeclamptic pregnancies. J. Soc. Gynecol. Investig. 12, 428–432 (2005).
De Wit, D. et al. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J. Autoimmun. 21, 277–281 (2003).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
Among the funding sources to the author's laboratory is research support from XOMA (U.S.) L.L.C. that manufactures recombinant BPI.
Related links
Glossary
- Hygiene hypothesis
-
The theory that exposure to microbial components, including Toll-like receptor (TLR) agonists during the neonatal, infancy and early-childhood phases of development serves to polarize the immune response towards T helper 1 (TH1)-cell, and away from TH2-cell, responses, thereby reducing the likelihood of allergy and/or atopy. Consistent with this hypothesis, there are inverse epidemiological relationships between the rates of infection and autoimmunity — for example, as the rates of common infections have dropped in wealthy industrialized countries, the rates of allergy and autoimmune disease have risen.
- Erythema toxicum neonatorum
-
A common, transient and benign rash of the newborn, characterized by raised lesions on an erythematous base. Recent evidence reveals that erythema toxicum probably reflects an innate immune response to initial skin colonization by Gram-positive bacteria (for example, coagulase-negative staphylococci) that penetrate neonatal skin through hair follicles. This bacterial penetration is associated with the activation of tissue macrophages and production of interleukin-1 (IL-1) and IL-6, which are capable of contributing to a systemic acute-phase response.
- Crypts
-
Tubular invaginations of the intestinal epithelium. Paneth cells are found at the base of the crypts and produce antimicrobial proteins and peptides, including phospholipase A2 and defensins, as well as stem cells, which continuously divide and are the source of all intestinal epithelial cells. Villi are projections into the lumen and have an outer layer of cells that mainly consists of mature, absorptive enterocytes but also contain mucus-secreting goblet cells.
- Necrotizing enterocolitis
-
(NEC). A gastrointestinal disease predominantly affecting premature low-birth-weight infants. NEC involves infection and inflammation that causes destruction of the intestine. Although the pathophysiology of NEC is not yet completely defined, increasing evidence indicates that immaturity of intestinal innate immune function of the premature gut, characterized by over-exuberant interleukin-8 responses of intestinal epithelial cells to lipopolysaccharide, is a major factor.
- Chorioamnionitis
-
Infection of the placental tissues and amniotic fluid as a result of ascending infection by bacteria that can be present in the vagina, such as Escherichia coli and group B Streptococcus. Chorioamnionitis can cause maternal bacteremia and can lead to pre-term delivery and neonatal infection.
- Bronchopulmonary dysplasia
-
(BPD). A disease of the respiratory system that is most frequent in pre-term infants, and is characterized by inflammation and scarring of the lungs resulting in abnormal development of lung tissue. Recent evidence shows that Toll-like-receptor-mediated inflammatory responses can reproduce the pulmonary histological findings characteristic of BPD, raising the possibility that innate immune responses may contribute to its pathophysiology.
- Cerebral palsy
-
A disorder of posture and movement due to damage to motor areas of the brain, often associated with a history of perinatal complications. The pathophysiology of cerebral palsy is still under investigation, but recent evidence indicates that a combination of hypoxic–ischemic insult and Toll-like-receptor-mediated inflammation can synergistically trigger neurodegeneration.
Rights and permissions
About this article
Cite this article
Levy, O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol 7, 379–390 (2007). https://doi.org/10.1038/nri2075
Issue Date:
DOI: https://doi.org/10.1038/nri2075