Key Points
-
The anatomical relationship between the placenta and the uterus holds the key to our understanding of the 'immunological paradox' of pregnancy because this is where direct tissue contact occurs.
-
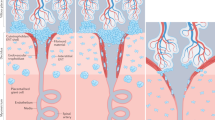
There is great diversity in placental structures in eutherian mammals. For immunologists, the most important feature is the extent to which the placental trophoblast cells invade the uterus. This ranges from no invasion at all (epitheliochorial placentation) to very extensive invasion (haemochorial placentation). The human placenta is the most invasive of all.
-
During pregnancy, in invasive forms of placentation, the uterine lining is transformed into decidual tissue. The most obvious feature of the decidua is the influx of a distinctive population of uterine natural killer (NK) cells.
-
Trophoblast cells express an array of MHC molecules some of which might be potential ligands for receptors expressed by the NK cells (CD94–NKG2A and KIRs (killer-cell immunoglobulin-like receptors)) and expressed by myelomonocytic cells (LILRB1 (leukocyte immunoglobulin-like receptor B1) and LILRB2) in the uterus. Interaction between HLA-C expressed by trophoblast cells and KIRs on maternal NK cells influences reproductive performance. Binding of HLA-G to LILRB molecules might induce tolerance in maternal T cells, thereby allowing cooperation between the innate and adaptive immune systems in mammalian reproduction.
-
It is proposed that the function of uterine NK cells is to alter the structure of the uterine spiral arteries that supply the feto-placental unit. This effect could be mediated directly by affecting the structure or function of the vessel wall (as in mice) or indirectly through the influence on trophoblast-cell infiltration. The arterial modification is necessary to allow sufficient blood flow to the placenta and fetus. Inadequate arterial transformation results in pregnancy disorders (such as fetal growth restriction or pre-eclampsia).
Abstract
The traditional way to study the immunology of pregnancy follows the classical transplantation model, which views the fetus as an allograft. A more recent approach, which is the subject of this Review, focuses on the unique, local uterine immune response to the implanting placenta. This approach requires knowledge of placental structure and its variations in different species, as this greatly affects the type of immune response that is generated by the mother. At the implantation site, cells from the mother and the fetus intermingle during pregnancy. Unravelling what happens here is crucial to our understanding of why some human pregnancies are successful whereas others are not.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Medawar, P. B. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp. Soc. Exp. Biol. 7, 320–338 (1953). The seminal paper that introduced the concept of the fetus as an allograft.
Trowsdale, J. & Betz, A. G. Mother's little helpers: mechanisms of maternal-fetal tolerance. Nature Immunol. 7, 241–246 (2006).
Steven, D. H. (ed) Comparative Placentation (Academic Press, New York, 1975).
Mossman, H. W. Vertebrate fetal membranes. (Rutgers University Press, New Brunswick, 1987).
Wooding, F. B. P. & Flint, A. P. F. in Marshall's Physiology of Reproduction Vol. 3 Ch. 4 (ed. Lamming, G. E.) 230–466 (Chapman and Hall, London, 1994).
Moffett-King, A. Natural killer cells and pregnancy. Nature Rev. Immunol. 2, 656–663 (2002).
Johnson, M. Origins of pluriblast and trophoblast in the eutherian conceptus. Reprod. Fertil. Dev. 8, 699–709 (1996).
Moffett, A., Loke, Y. W. & McLaren, A. The Biology and Pathology of Trophoblast (Cambridge University Press, Cambridge, 2006).
Blackburn, D. G. Reconstructing the evolution of viviparity and placentation. J. Theor. Biol. 192, 183–190 (1998).
Romer, A. S. Major steps in vertebrate evolution. Science 158, 1629–1637 (1967).
Luckett, W. P. in Major Patterns of Vertebrate Evolution (eds Hecht, M. K., Goody, P. C. & Hecht, B. M.) 439–516 (Plenum Press, New York 1977).
Hubrecht, A. A. W. Studies in mammalian embryology. 1. The placentation of Erinaceus europaeus, with remarks on the phylogeny of the placenta. Q. J. Microsc. Sci. 119, 283–404 (1889).
Amoroso, E. C. in Marshall's Physiology of Reproduction Vol. 2 Ch. 15 (ed.Parkes, A. S.) 127–311 (Longman, Green and Co., London, 1952).
Vogel, P. The current molecular phylogeny of Eutherian mammals challenges previous interpretations of placental evolution. Placenta 26, 591–596 (2005).
Murphy, W. J. et al. Resolution of the early placental mammal radiation using Bayesian phylogenetics. Science 294, 2348–2351 (2001).
Kriegs, J. O. et al. Retroposed elements as archives for the evolutionary history of placental mammals. PLoS Biol. 4, e91 (2006).
Mess, A. & Carter, A. M. Evolutionary transformations of fetal membrane characters in Eutheria with special reference to Afrotheria. J. Exp. Zoolog. B. Mol. Dev. Evol. 306, 140–163 (2006).
Enders, A. C. & Carter, A. M. What can comparative studies of placental structure tell us? — A review. Placenta 25 (Suppl. A), S3–S9 (2004).
Wildman, D. E. et al. Evolution of the mammalian placenta revealed by phylogenetic analysis. Proc. Natl Acad. Sci. USA 103, 3203–3208 (2006).
Clarke, C. A. Prevention of rhesus iso-immunisation. Lancet 2, 1–7 (1968).
Nelson, J. L. Maternal-fetal immunology and autoimmune disease. Is some autoimmune disease auto-allo or allo-auto immune? Arthritis Rheum. 39, 191–194 (1996).
Pijnenborg, R., Vercruysse, L. & Hanssens, M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 17 February 2006 [epub ahead of print]. The definitive paper of the placental bed based on a life-time's study.
Jauniaux, E., Poston, L. & Burton, G. J. Placental-related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Hum. Reprod. Update 8 May 2006 [epub ahead of print].
King, A. et al. Uterine leukocytes and decidualization. Hum. Reprod. Update 6, 28–36 (2000).
Robertson, W. B. in Obstetrical and Gynaecological Pathology (ed. Fox, H.) 1149–1176 (Churchill Livingstone, 1987).
Kirby, D. R. S. in The Early Conceptus, Normal and Abnormal (ed. Park, W. W.) 68–73 (Proceedings of Symposium, Queen's College, Dundee, 1965).
Khong, T. Y. & Robertson, W. B. Placenta creta and placenta praevia creta. Placenta 8, 399–409 (1987).
McLaren, A. in The Early Conceptus, Normal and Abnormal (ed. Park, W. W.) 27–33 (Proceedings of Symposium, Queen's College, Dundee, 1965).
Martin, R. D. Human reproduction: a comparative background for medical hypotheses. J. Reprod. Immunol. 59, 111–135 (2003).
Ramsey, E. M., Houston, M. L. & Harris, J. W. S. Interactions of the trophoblast and maternal tissues in three closely related primate species. Am. J. Obstet. Gynecol. 124, 647–652 (1976). This paper emphasizes that, even among closely related species of primates, the ways trophoblast cells interact with maternal tissues show many differences.
Rockwell, L. C., Vargas, E. & Moore, L. G. Human physiological adaptation to pregnancy: inter- and intraspecific perspectives. Am. J. Hum. Biol. 15, 330–341 (2003).
Chaline, J. Increased cranial capacity in hominoid evolution and preeclampsia. J. Reprod. Immunol. 59, 137–152 (2003).
Baker, J. M., Bamford, A. I. & Antczak, D. F. Modulation of allospecific CTL responses during pregnancy in equids: an immunological barrier to interspecies matings? J. Immunol. 162, 4496–4501 (1999).
Davies, C. J., Eldridge, J. A., Fisher, P. J. & Schlafer, D. H. Evidence for expression of both classical and non-classical major histocompatibility complex class I genes in bovine trophoblast cells. Am. J. Reprod. Immunol. 55, 188–200 (2006).
Bainbridge, D. R., Sargent, I. L. & Ellis, S. A. Increased expression of major histocompatibility complex (MHC) class I transplantation antigens in bovine trophoblast cells before fusion with maternal cells. Reproduction 122, 907–913 (2001).
Stewart, I. J. Granulated metrial gland cells in 'minor' species. J. Reprod. Immunol. 40, 129–146 (1998).
Georgiades, P., Ferguson-Smith, A. C. & Burton, G. J. Comparative developmental anatomy of the murine and human definitive placentae. Placenta 23, 3–19 (2002).
Ain, R., Canham, L. N. & Soares, M. J. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev. Biol. 260, 176–190 (2003).
Vercruysse, L., Caluwaerts, S., Luyten, C. & Pijnenborg, R. Interstitial trophoblast invasion in the decidua and mesometrial triangle during the last third of pregnancy in the rat. Placenta 27, 22–33 (2006).
Hemberger, M., Nozaki, T., Masutani, M. & Cross, J. C. Differential expression of angiogenic and vasodilatory factors by invasive trophoblast giant cells depending on depth of invasion. Dev. Dyn. 227, 185–191 (2003).
Croy, B. A., Chantakru, S., Esadeg, S., Ashkar, A. A. & Wei, Q. Decidual natural killer cells: key regulators of placental development (a review). J. Reprod. Immunol. 57, 151–168 (2002). Comprehensive review discussing uterine NK cells in mice.
Tafuri, A., Alferink, J., Moller, P., Hammerling, G. J. & Arnold, B. T cell awareness of paternal alloantigens during pregnancy. Science 270, 630–633 (1995).
Redline, R. W. & Lu, C. Y. Localization of fetal major histocompatibility complex antigens and maternal leukocytes in murine placenta. Implications for maternal-fetal immunological relationship. Lab. Invest. 61, 27–36 (1989).
Zuckermann, F. A. & Head, J. R. Expression of MHC antigens on murine trophoblast and their modulation by interferon. J. Immunol. 137, 846–853 (1986).
Erlebacher, A., Lukens, A. K. & Glimcher, L. H. Intrinsic susceptibility of mouse trophoblasts to natural killer cell-mediated attack in vivo. Proc. Natl Acad. Sci. USA 99, 16940–16945 (2002).
Munn, D. H. et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281, 1191–1193 (1998).
Erlebacher, A., Zhang, D., Parlow, A. F. & Glimcher, L. H. Ovarian insufficiency and early pregnancy loss induced by activation of the innate immune system. J. Clin. Invest. 114, 39–48 (2004).
Aluvihare, V. R. et al. Regulatory T cells mediate maternal tolerance to the fetus. Nature Immunol. 5, 266–271 (2004). Paper describing a potential role for regulatory T cells in allogeneic murine pregnancies.
Sollwedel, A. et al. Protection from abortion by heme oxygenase-1 up-regulation is associated with increased levels of Bag-1 and neuropilin-1 at the fetal-maternal interface. J. Immunol. 175, 4875–4885 (2005).
Zhu, X. Y. et al. Blockade of CD86 signaling facilitates a Th2 bias at the maternal-fetal interface and expands peripheral CD4+CD25+ regulatory T cells to rescue abortion-prone fetuses. Biol. Reprod. 72, 338–345 (2005).
Zenclussen, A. C. et al. Regulatory T cells induce a privileged tolerant microenvironment at the fetal-maternal interface. Eur. J. Immunol. 36, 82–94 (2006).
Xu, C. et al. A critical role for murine complement regulator crry in fetomaternal tolerance. Science 287, 498–501 (2000).
Hunt, J. S., Vassmer, D., Ferguson, T. A. & Miller, L. Fas ligand is positioned in mouse uterus and placenta to prevent trafficking of activated leukocytes between the mother and the conceptus. J. Immunol. 158, 4122–4128 (1997).
Guleria, I. et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J. Exp. Med. 202, 231–237 (2005).
Krishnan, L., Guilbert, L. J., Wegmann, T. G., Belosevic, M. & Mosmann, T. R. T helper 1 response against Leishmania major in pregnant C57BL/6 mice increases implantation failure and fetal resorptions. Correlation with increased IFN-γ and TNF and reduced IL-10 production by placental cells. J. Immunol. 156, 653–662 (1996).
Fallon, P. G. et al. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity 17, 7–17 (2002).
Baban, B. et al. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J. Reprod. Immunol. 61, 67–77 (2004).
Somerset, D. A., Zheng, Y., Kilby, M. D., Sansom, D. M. & Drayson, M. T. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+CD4+ regulatory T-cell subset. Immunology 112, 38–43 (2004).
Sasaki, Y. et al. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol. Hum. Reprod. 10, 347–353 (2004).
Grabowska, A. Placental antigenicity and maternal immunoregulation in human and murine pregnancy. Ph.D. Thesis, Univ. Cambridge (1989).
Ostensen, M. Sex hormones and pregnancy in rheumatoid arthritis and systemic lupus erythematosus. Ann. NY Acad. Sci. 876, 131–144 (1999).
Buyon, J. P. The effects of pregnancy on autoimmune diseases. J. Leukoc. Biol. 63, 281–287 (1998).
Gardner, L. & Moffett, A. Dendritic cells in the human decidua. Biol. Reprod. 69, 1438–1446 (2003).
Shao, L., Jacobs, A. R., Johnson, V. V. & Mayer, L. Activation of CD8+ regulatory T cells by human placental trophoblasts. J. Immunol. 174, 7539–7547 (2005).
Shiroishi, M. et al. Efficient leukocyte Ig-like receptor signaling and crystal structure of disulfide-linked HLA-G dimer. J. Biol. Chem. 281, 10439–10447 (2006).
Chang, C. C. et al. Tolerization of dendritic cells by TS cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nature Immunol. 3, 237–243 (2002).
Velten, F. W., Duperrier, K., Bohlender, J., Metharom, P. & Goerdt, S. A gene signature of inhibitory MHC receptors identifies a BDCA3+ subset of IL-10-induced dendritic cells with reduced allostimulatory capacity in vitro. Eur. J. Immunol. 34, 2800–2811 (2004).
Ristich, V., Liang, S., Zhang, W., Wu, J. & Horuzsko, A. Tolerization of dendritic cells by HLA-G. Eur. J. Immunol. 35, 1133–1142 (2005).
Sherman, M. E., Mazur, M. T. & Kurman, R. J. in Blaustain's Pathology of the Female Genital Tract (ed. Kurman, R. J.) 428 (Springer, New York, 2002).
Ryan, A. F., Grendell, R. L., Geraghty, D. E. & Golos, T. G. A soluble isoform of the rhesus monkey nonclassical MHC class I molecule Mamu-AG is expressed in the placenta and the testis. J. Immunol. 169, 673–683 (2002).
Stern, P. L. et al. Class I-like MHC molecules expressed by baboon placental syncytiotrophoblast. J. Immunol. 138, 1088–1091 (1987).
King, A., et al. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur. J. Immunol. 30, 1623–1631 (2000).
Rajagopalan, S. et al. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS. Biol. 4, e9 (2006). A careful study describing stimulation of NK cells by soluble HLA-G that is endocytosed by KIR2DL4.
Redman, C. W. & Sargent, I. L. Preeclampsia and the systemic inflammatory response. Semin. Nephrol. 24, 565–570 (2004).
Parham, P. MHC class I molecules and KIRs in human history, health and survival. Nature Rev. Immunol. 5, 201–214 (2005). A comprehensive reference covering all aspects of KIRs.
Hiby, S. E. et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J. Exp. Med. 200, 957–965 (2004). This paper provides genetic evidence for a role for NK cells in regulating trophoblast-cell invasion.
Verma, S., King, A. & Loke, Y. W. Expression of killer-cell inhibitory receptors (KIR) on human uterine NK cells. Eur. J. Immunol. 27, 979–983 (1997).
Martin, M. P. & Carrington, M. Immunogenetics of viral infections. Curr. Opin. Immunol. 17, 510–516 (2005).
Abi-Rached, L. & Parham, P. Natural selection drives recurrent formation of activating killer cell immunoglobulin-like receptor and Ly49 from inhibitory homologues. J. Exp. Med. 201, 1319–1332 (2005).
LaBonte, M. L., Hershberger, K. L., Korber, B. & Letvin, N. L. The KIR and CD94/NKG2 families of molecules in the rhesus monkey. Immunol. Rev. 183, 25–40 (2001).
Guethlein, L. A., Flodin, L. R., Adams, E. J. & Parham, P. NK cell receptors of the orangutan (Pongo pygmaeus): a pivotal species for tracking the coevolution of killer cell Ig-like receptors with MHC-C. J. Immunol. 169, 220–229 (2002).
Rajalingam, R., Parham, P. & Abi-Rached, L. Domain shuffling has been the main mechanism forming new hominoid killer cell Ig-like receptors. J. Immunol. 172, 356–369 (2004).
Khakoo, S. I. et al. Rapid evolution of NK cell receptor systems demonstrated by comparison of chimpanzees and humans. Immunity 12, 687–698 (2000).
Emes, R. D., Goodstadt, L., Winter, E. E. & Ponting, C. P. Comparison of the genomes of human and mouse lays the foundation of genome zoology. Hum. Mol. Genet. 12, 701–709 (2003).
Selwood, L. & Johnson, M. H. Trophoblast and hypoblast in the monotreme, marsupial and eutherian mammal: evolution and origins. BioEssays 28, 128–145 (2006).
Koopman, L. A. et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J. Exp. Med. 198, 1201–1212 (2003).
Bulmer, J. N. & Lash, G. E. Human uterine natural killer cells: a reappraisal. Mol. Immunol. 42, 511–521 (2005).
Li, X. F. et al. Angiogenic growth factor messenger ribonucleic acids in uterine natural killer cells. J. Clin. Endocrinol. Metab. 86, 1823–1834 (2001).
Moffett, A., Regan, L. & Braude, P. Natural killer cells, miscarriage, and infertility. BMJ 329, 1283–1285 (2004).
Pijnenborg, R., Robertson, W. B. & Brosens, I. The arterial migration of trophoblast in the uterus of the golden hamster, Mesocricetus auratus. J. Reprod. Fertil. 40, 269–280 (1974).
Nanaev, A. et al. Physiological dilation of uteroplacental arteries in the guinea pig depends on nitric oxide synthase activity of extravillous trophoblast. Cell Tissue Res. 282, 407–421 (1995).
Pijnenborg, R., Robertson, W. B., Brosens, I. & Dixon, G. Trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta 2, 71–91 (1981).
Finn, C. Menstruation: a nonadaptive consequence of uterine evolution. Q. Rev. Biol. 73, 163–173 (1998).
Blankenship, T. N. & Enders, A. C. Modification of uterine vasculature during pregnancy in macaques. Microsc. Res. Tech. 60, 390–401 (2003).
Pijnenborg, R., D'Hooghe, T., Vercruysse, L. & Bambra, C. Evaluation of trophoblast invasion in placental bed biopsies of the baboon, with immunohistochemical localisation of cytokeratin, fibronectin and laminin. J. Med. Primatol. 25, 272–281 (1996).
Acknowledgements
The authors thank D. Antczak, G. Burton, S. Ellis, S. Murphy, P. Parham, R. Pijneneborg, A. Sharkey and P. Wooding for helpful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Trophoblast cells
-
Trophoblast cells are the earliest extra-embryonic cells to differentiate from the cells of the mammalian embryo. They surround the conceptus throughout gestation and are in direct contact with maternal tissues.
- Blastocyst
-
After fertilization, the potential embryo undergoes mitotic division and, at the 128-cell stage in humans, two distinct cell lineages are present. Trophoblast cells are derived from the trophectoderm that surrounds the blastocyst and the inner cell mass gives rise to the embryo.
- Eutherian placenta
-
Eutherian mammals include all mammalian species except marsupials and egg-laying monotremes. The eutherian placenta is well developed compared with the marsupial placenta and has a great diversity of forms.
- Amniote egg
-
Eggs of amniote vertebrates provide an interface between the embryo and its immediate environment, therefore allowing increased respiratory and excretory capacity as well as nutrient provision.
- Yolk sac
-
The first of the four extra-embryonic membranes of amniote eggs to form during embryogenesis. It surrounds the mass of yolk in reptile and bird eggs and is connected to the midgut by the yolk stalk. The yolk sac is also formed in mammals, despite the absence of yolk.
- Amnion
-
The innermost membranous sac of amniote eggs. It is filled with a serous fluid and encloses the embryo of an amniote (reptile, bird or mammal).
- Chorion
-
In birds and reptiles, the chorion adheres to the shell and is highly vascularized to function in gas exchange. In mammals, it forms the fetal contribution to the placenta, made by an outer layer of trophoblast cells and inner layer of extra-embryonic mesoderm, which contains blood vessels that allow exchange of materials with the maternal circulation.
- Allantois
-
The extra-embryonic membrane that emerges as a sac from the posterior part of hindgut of the embryo. It fuses with the chorion to form the chorio-allantoic placenta. The connection it makes between the embryo and the placenta becomes the umbilical cord.
- Retroposed elements
-
Retroposons randomly insert into the genomes with little likelihood of the same element integrating into the orthologous position in different species. Analysis of the patterns of presence or absence of retroposons is a reliable method for studying the evolutionary history of organisms.
- Convergent evolution
-
The process whereby organisms that are not closely related independently acquire similar characteristics while evolving in separate and sometimes varying ecosystems.
- Haemolytic disease of the newborn
-
If there is rhesus-blood-group incompatibility between the mother and her fetus, the mother makes an antibody response against fetal red blood cells that access the mother's circulation at delivery. These IgG antibodies cross the placenta during a subsequent pregnancy, which results in the destruction of fetal red blood cells, leading to haemolytic disease of the new born.
- Maternal and fetal microchimerism
-
The presence of fetal cells in the mother or maternal cells in the fetus. Fetal or maternal cells generally cross the placenta at delivery and might persist for many years.
- Systemic sclerosis
-
A chronic autoimmune disease that causes a hardening of the skin. The skin thickens because of increased deposits of collagen. Compared with the localized form of the disease (scleroderma), systemic sclerosis causes more widespread skin changes and can be associated with damage to the lungs, heart and kidneys.
- Endometriotic foci
-
Foci of endometrial tissue outside the endometrium or myometrium (muscle wall) of the uterus. They are usually found in the peritoneum.
- Tubal pregnancy
-
An ectopic pregnancy occurs when the blastocyst implants at a site outside the uterus. Most ectopic pregnancies occur in the fallopian tube so the terms ectopic pregnancy and tubal pregnancy are nearly synonymous.
- Placenta creta
-
A condition when placental trophoblast cells invade deeply into the muscle coat (myometrium) of the uterus, usually because of the absence of decidua. This can lead to uterine rupture, torrential haemorrhage and failure of the placenta to separate after delivery.
- Procrustean bed
-
In Greek mythology, Procrustes (whose name means he who stretches) was a host who adjusted his guests to their bed. If they were longer than the bed, he cut off the redundant part; if shorter, he stretched them till they fitted it. Any attempt to reduce men to one standard, one way of thinking or one way of acting, is called placing them on a Procrustean bed.
- Pre-eclampsia
-
Eclampsia (in Greek meaning bolt from the blue) describes grand mal seizures (epileptic fits) occurring towards the end of pregnancy. Pre-eclampsia describes the symptoms that precede eclampsia, which include oedema, proteinuria and hypertension.
- Inferior vena cava
-
The large vein that carries de-oxygenated blood from the lower half of the body to the heart.
- Endometrial cups
-
A focal collection of trophoblast cells that penetrate the uterus of horses. These cells are responsible for secretion of equine chorionic gonadotrophin.
- Ectoplacental cone
-
A core of rapidly dividing trophoblast cells with an outer layer of giant cells that is present in the developing mouse conceptus at 7.5 days post-coitum.
- Syncytiotrophoblast
-
The outermost trophoblast-cell layer covering the chorionic villi that is formed by fusion of the underlying layer of mononuclear trophoblast cells to become a multinucleated syncytium, which forms a barrier between the fetus and the mother.
Rights and permissions
About this article
Cite this article
Moffett, A., Loke, C. Immunology of placentation in eutherian mammals. Nat Rev Immunol 6, 584–594 (2006). https://doi.org/10.1038/nri1897
Issue Date:
DOI: https://doi.org/10.1038/nri1897
This article is cited by
-
Quercetin stimulates trophoblast fusion via the mitochondrial function
Scientific Reports (2024)
-
Centromeric AA motif in KIR as an optimal surrogate marker for precision definition of alloimmune reproductive failure
Scientific Reports (2024)
-
Testing effects of partner support and use of oral contraception during relationship formation on severity of nausea and vomiting in pregnancy
BMC Pregnancy and Childbirth (2023)
-
Potential Therapeutic Effects of Human Amniotic Epithelial Cells on Gynecological Disorders Leading to Infertility or Abortion
Stem Cell Reviews and Reports (2023)
-
MiR-3074-5p Regulates Trophoblasts Function via EIF2S1/GDF15 Pathway in Recurrent Miscarriage
Reproductive Sciences (2023)