Key Points
-
Dectin-1 is a mainly myeloid-cell-expressed NK-cell-receptor-like C-type lectin that functions as a transmembrane pattern-recognition receptor through its ability to bind β-glucan carbohydrates. Dectin-1 also recognizes an unidentified endogenous ligand on T cells, possibly acting as a co-stimulatory molecule.
-
Following ligand binding, dectin-1 can mediate various cellular responses, including cytokine and chemokine production, the respiratory burst and phagocytosis.
-
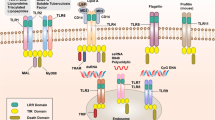
Cellular responses are mediated by signalling events initiated from the atypical cytoplasmic immunoreceptor tyrosine-based activation motif of dectin-1 using novel pathways, including the protein spleen tyrosine kinase (SYK) and collaborative signalling with the Toll-like receptors, in a cell-specific manner.
-
Dectin-1 can recognize several fungal pathogens and might play a role in the innate response to these organisms. These pathogens, in turn, might have mechanisms for avoiding recognition by this receptor.
-
Dectin-1 is likely to play a role in the protective effects against infectious and non-infectious diseases exerted by purified soluble β-glucans in vivo, although the mechanisms behind these activities are unclear.
-
On certain genetic backgrounds, dectin-1 can play a central role in the development of β-glucan-induced autoimmune disease and might also contribute to the development of fungal-induced respiratory disorders.
-
The activities of dectin-1 might be representative of other pattern-recognition receptors, especially other myeloid-cell-expressed NK-cell-receptor-like C-type lectins, which have similar signalling motifs in their cytoplasmic tails.
Abstract
Dectin-1 is a natural killer (NK)-cell-receptor-like C-type lectin that is thought to be involved in innate immune responses to fungal pathogens. This transmembrane signalling receptor mediates various cellular functions, from fungal binding, uptake and killing, to inducing the production of cytokines and chemokines. These activities could influence the resultant immune response and can, in certain circumstances, lead to autoimmunity and disease. As I discuss here, understanding the molecular mechanisms behind these functions has revealed new concepts, including collaborative signalling with the Toll-like receptors (TLRs) and the use of spleen tyrosine kinase (SYK), that have implications for the role of other non-TLR pattern-recognition receptors in immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Janeway, C. A. Jr. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol. Today 13, 11?16 (1992).
Martinon, F. & Tschopp, J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 26, 447?454 (2005).
Gordon, S. Pattern recognition receptors: doubling up for the innate immune response. Cell 111, 927?930 (2002).
Doyle, S. E. et al. Toll-like receptors induce a phagocytic gene program through p38. J. Exp. Med. 199, 81?90 (2004).
Blander, J. M. & Medzhitov, R. Regulation of phagosome maturation by signals from toll-like receptors. Science 304, 1014?1018 (2004).
Ezekowitz, R. A., Sastry, K., Bailly, P. & Warner, A. Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J. Exp. Med. 172, 1785?1794 (1990).
Brown, E. J. Complement receptors and phagocytosis. Curr. Opin. Immunol. 3, 76?82 (1991).
Peiser, L., Gough, P. J., Kodama, T. & Gordon, S. Macrophage class A scavenger receptor-mediated phagocytosis of Escherichia coli: role of cell heterogeneity, microbial strain, and culture conditions in vitro. Infect. Immun. 68, 1953?1963 (2000).
Elomaa, O. et al. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell 80, 603?609 (1995).
Herre, J. et al. Dectin-1 utilizes novel mechanisms for yeast phagocytosis in macrophages. Blood 104, 4038?4045 (2004).
Gantner, B. N., Simmons, R. M., Canavera, S. J., Akira, S. & Underhill, D. M. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197, 1107?1117 (2003).
Underhill, D. M., Rossnagle, E., Lowell, C. A. & Simmons, R. M. Dectin-1 activates SYK tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 106, 2543?2550 (2005).
Goldstein, I. M., Roos, D., Kaplan, H. B. & Weissmann, G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J. Clin. Invest. 56, 1155?1163 (1975).
Wright, S. D. & Silverstein, S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J. Exp. Med. 158, 2016?2023 (1983).
Hoebe, K. et al. CD36 is a sensor of diacylglycerides. Nature 433, 523?527 (2005).
Jiang, Z. et al. CD14 is required for MyD88-independent LPS signaling. Nature Immunol. 6, 565?570 (2005). References 15 and 16 show that non-TLR PRRs can contribute to inflammatory responses by the presentation of PAMPS.
Swain, S. D., Lee, S. J., Nussenzweig, M. C. & Harmsen, A. G. Absence of the macrophage mannose receptor in mice does not increase susceptibility to Pneumocystis carinii infection in vivo. Infect. Immun. 71, 6213?6221 (2003).
Lee, S. J., Zheng, N. Y., Clavijo, M. & Nussenzweig, M. C. Normal host defense during systemic candidiasis in mannose receptor-deficient mice. Infect. Immun. 71, 437?445 (2003).
Brown, G. D. et al. dectin-1 mediates the biological effects of β-glucan. J. Exp. Med. 197, 1119?1124 (2003). This article, together with reference 11, shows that signalling from dectin-1 directly contributes to the inflammatory response.
Yokoyama, W. M. et al. cDNA cloning of mouse NKR-P1 and genetic linkage with LY-49. Identification of a natural killer cell gene complex on mouse chromosome 6. J. Immunol. 147, 3229?3236 (1991).
Ariizumi, K. et al. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J. Biol. Chem. 275, 20157?20167 (2000).
Sawamura, T. et al. An endothelial receptor for oxidized low-density lipoprotein. Nature 386, 73?77 (1997).
Marshall, A. S. et al. Identification and characterization of a novel human myeloid inhibitory C-type lectin-like receptor (MICL) that is predominantly expressed on granulocytes and monocytes. J. Biol. Chem. 279, 14792?14802 (2004).
Colonna, M., Samaridis, J. & Angman, L. Molecular characterization of two novel C-type lectin-like receptors, one of which is selectively expressed in human dendritic cells. Eur. J. Immunol. 30, 697?704 (2000).
Sobanov, Y. et al. A novel cluster of lectin-like receptor genes expressed in monocytic, dendritic and endothelial cells maps close to the NK receptor genes in the human NK gene complex. Eur. J. Immunol. 31, 3493?3503. (2001).
Chen, M., Masaki, T. & Sawamura, T. LOX-1, the receptor for oxidized low-density lipoprotein identified from endothelial cells: implications in endothelial dysfunction and atherosclerosis. Pharmacol. Ther. 95, 89?100 (2002).
Brown, G. D. & Gordon, S. Immune recognition: A new receptor for β-glucans. Nature 413, 36?37 (2001).
Shimaoka, T. et al. LOX-1 supports adhesion of Gram-positive and Gram-negative bacteria. J. Immunol. 166, 5108?5114 (2001).
Delneste, Y. et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity 17, 353?362 (2002).
Oka, K. et al. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc. Natl. Acad. Sci. USA 95, 9535?9540 (1998).
Rogers, N. C. et al. Syk-dependent cytokine induction by dectin-1 reveals a novel pattern recognition pathway for C-type lectins. Immunity 22, 507?517 (2005). This paper, together with reference 12, is the first demonstration that signalling from C-type lectins occurs through SYK and that this can be mediated by a single YXXL motif.
Mason, L. H. et al. The Ly-49D receptor activates murine natural killer cells. J. Exp. Med. 184, 2119?2128 (1996).
Diefenbach, A. et al. Selective associations with signalling proteins determine stimulatory versus costimulatory activity of NKG2D. Nature Immunol. 3, 1142?1149 (2002).
Gilfillan, S., Ho, E. L., Cella, M., Yokoyama, W. M. & Colonna, M. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nature Immunol. 3, 1150?1155 (2002).
Hermanz-Falcon, P., Arce, I., Roda-Navarro, P. & Fernandez-Ruiz, E. Cloning of human DECTIN-1, a novel C-type lectin-like receptor gene expressed on dendritic cells. Immunogenetics 53, 288?295 (2001).
Willment, J. A., Gordon, S. & Brown, G. D. Characterisation of the human β-glucan receptor and its alternatively spliced isoforms. J. Biol. Chem. 276, 43818?43823 (2001).
Yokota, K., Takashima, A., Bergstresser, P. R. & Ariizumi, K. Identification of a human homologue of the dendritic cell-associated C-type lectin-1, dectin-1. Gene 272, 51?60 (2001).
Willment, J. A. et al. The human β-glucan receptor is widely expressed and functionally equivalent to murine dectin-1 on primary cells. Eur. J. Immunol. 35, 1539?1547 (2005).
Riedl, E., Tada, Y. & Udey, M. C. Identification and characterization of an alternatively spliced isoform of mouse Langerin/CD207. J. Invest. Dermatol. 123, 78?86 (2004).
Taylor, P. R. et al. The β-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 169, 3876?3882 (2002).
Reid, D. M. et al. Expression of the β-glucan receptor, dectin-1, on murine leukocytes in situ correlates with its function in pathogen recognition and reveals potential roles in leukocyte interactions. J. Leukoc. Biol. 76, 86?94 (2004).
Willment, J. A. et al. Dectin-1 expression and function is enhanced on alternatively activated and GM-CSF treated macrophages and negatively regulated by IL-10, dexamethasone and LPS. J. Immunol. 171, 4569?4573 (2003).
Weis, W. I., Taylor, M. E. & Drickamer, K. The C-type lectin superfamily in the immune system. Immunol. Rev. 163, 19?34 (1998).
Pavlicek, J. et al. Molecular characterization of binding of calcium and carbohydrates by an early activation antigen of lymphocytes CD69. Biochemistry 42, 9295?9306 (2003).
Gange, C. T. et al. Characterization of sugar binding by osteoclast inhibitory lectin. J. Biol. Chem. 279, 29043?29049 (2004).
Adachi, Y. et al. Characterization of β-glucan recognition site on C-type lectin, dectin 1. Infect. Immun. 72, 4159?4171 (2004).
Brown, G. D. et al. Dectin-1 is a major β-glucan receptor on macrophages. J. Exp. Med. 196, 407?412 (2002).
Grunebach, F., Weck, M. M., Reichert, J. & Brossart, P. Molecular and functional characterization of human dectin-1. Exp. Hematol. 30, 1309?1315 (2002).
Yokoyama, W. M. & Plougastel, B. F. Immune functions encoded by the natural killer gene complex. Nature Rev. Immunol. 3, 304?316 (2003).
Iizuka, K., Naidenko, O. V., Plougastel, B. F., Fremont, D. H. & Yokoyama, W. M. Genetically linked C-type lectin-related ligands for the NKRP1 family of natural killer cell receptors. Nature Immunol. 4, 801?807 (2003).
Steele, C. et al. Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the dectin-1 β-glucan recptor. J. Exp. Med. 198, 1677?1688 (2003).
Underhill, D. M. et al. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401, 811?815 (1999). This is the first paper showing the involvement of TLR2 in the inflammatory response to zymosan.
Ozinsky, A. et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. USA 97, 13766?13771 (2000).
Kataoka, K., Muta, T., Yamazaki, S. & Takeshige, K. Activation of macrophages by linear (1, 3)-β-D-glucans. J. Biol. Chem. 277, 36825?36831 (2002).
Young, S. H., Ye, J., Frazer, D. G., Shi, X. & Castranova, V. Molecular mechanism of tumor necrosis factor-α production in 1,3-β-glucan (zymosan)-activated macrophages. J. Biol. Chem. 276, 20781?20787 (2001).
Lanier, L. L., Corliss, B. C., Wu, J., Leong, C. & Phillips, J. H. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature 391, 703?707 (1998).
Van den Herik-Oudijk, I. E., Capel, P. J., van der Bruggen, T. & Van de Winkel, J. G. Identification of signaling motifs within human Fcg RIIa and Fcg RIIb isoforms. Blood 85, 2202?2211 (1995).
Pitcher, L. A. & van Oers, N. S. T-cell receptor signal transmission: who gives an ITAM? Trends Immunol. 24, 554?560 (2003).
Crowley, M. T. et al. A critical role for Syk in signal transduction and phagocytosis mediated by Fcγ receptors on macrophages. J. Exp. Med. 186, 1027?1039 (1997).
Turner, M. et al. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature 378, 298?302 (1995).
Cambi, A. & Figdor, C. G. Dual function of C-type lectin-like receptors in the immune system. Curr. Opin. Cell. Biol. 15, 539?546 (2003).
Curtis, B. M., Scharnowske, S. & Watson, A. J. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 89, 8356?8360 (1992).
Geijtenbeek, T. B. et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197, 7?17 (2003). This article shows that C-type lectin signalling can suppress TLR-mediated inflammatory responses.
Arbibe, L. et al. Toll-like receptor 2-mediated NF-κB activation requires a Rac1-dependent pathway. Nature Immunol. 1, 533?540 (2000).
Shimazu, R. et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189, 1777?1782 (1999).
Mantegazza, A. R. et al. CD63 Tetraspanin slows down cell migration and translocates to the endosomal/lysosomal/MIICs route after extracellular stimuli in human immature dendritic cells. Blood 104, 1183?1190 (2004).
Yoshitomi, H. et al. A role for fungal β-glucans and their receptor dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J. Exp. Med. 201, 949?960 (2005). This is the first report of a non-TLR PRR directly contributing to the development of autoimmunity.
Romani, L. Immunity to fungal infections. Nature Rev. Immunol. 4, 11?24 (2004).
Klis, F. M., Mol, P., Hellingwerf, K. & Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 26, 239?256 (2002).
Gantner, B. N., Simmons, R. M. & Underhill, D. M. dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 24, 1277?1286 (2005). This article shows that fungi can avoid recognition by dectin-1 by masking their β-glucan.
Torosantucci, A. et al. A novel glyco-conjugate vaccine against fungal pathogens. J. Exp. Med. 202, 597?606 (2005).
Viriyakosol, S., Fierer, J., Brown, G. D. & Kirkland, T. N. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and dectin-1. Infect. Immun. 73, 1553?1560 (2005).
Vazquez-Torres, A., Jones-Carson, J., Wagner, R. D., Warner, T. & Balish, E. Early resistance of interleukin-10 knockout mice to acute systemic candidiasis. Infect. Immun. 67, 670?674 (1999).
Belkaid, Y. & Rouse, B. T. Natural regulatory T cells in infectious disease. Nature Immunol. 6, 353?360 (2005).
Netea, M. G. et al. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J. Immunol. 172, 3712?3718 (2004).
Montagnoli, C. et al. B7/CD28-dependent CD4+CD25+ regulatory T cells are essential components of the memory-protective immunity to Candida albicans. J. Immunol. 169, 6298?6308 (2002).
Bellocchio, S. et al. The contribution of the toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J. Immunol. 172, 3059?3069 (2004). A comprehensive study of the role of selected TLRs in fungal infection, using knockout mice.
Villamon, E. et al. Toll-like receptor-2 is essential in murine defenses against Candida albicans infections. Microbes Infect. 6, 1?7 (2004).
Netea, M. G., Van der Meer, J. W. & Kullberg, B. J. Toll-like receptors as an escape mechanism from the host defense. Trends Microbiol. 12, 484?488 (2004).
Gale, C. A. et al. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science 279, 1355?1358 (1998).
Lo, H. J. et al. Nonfilamentous C. albicans mutants are avirulent. Cell 90, 939?949 (1997).
Gow, N. A., Brown, A. J. & Odds, F. C. Fungal morphogenesis and host invasion. Curr. Opin. Microbiol. 5, 366?371 (2002).
d'Ostiani, C. F. et al. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 191, 1661?1674 (2000). This paper describes the different response of DCs to yeast and hyphal forms of C. albicans.
Cross, C. E. & Bancroft, G. J. Ingestion of acapsular Cryptococcus neoformans occurs via mannose and β-glucan receptors, resulting in cytokine production and increased phagocytosis of the encapsulated form. Infect. Immun. 63, 2604?2611 (1995).
Borges-Walmsley, M. I., Chen, D., Shu, X. & Walmsley, A. R. The pathobiology of Paracoccidioides brasiliensis. Trends Microbiol. 10, 80?87 (2002).
Williams, D. L. et al. Pre-clinical safety evaluation of soluble glucan. Int. J. Immunopharmacol. 10, 405?414 (1988).
Adams, D. S. et al. PGG-Glucan activates NF-κB-like and NF-IL-6-like transcription factor complexes in a murine monocytic cell line. J. Leukoc. Biol. 62, 865?873 (1997).
Battle, J. et al. Ligand binding to the (1→3)-β-D-glucan receptor stimulates NFκB activation, but not apoptosis in U937 cells. Biochem. Biophys. Res. Commun. 249, 499?504 (1998).
Williams, D. L. et al. Modulation of the phosphoinositide 3-kinase pathway alters innate resistance to polymicrobial sepsis. J. Immunol. 172, 449?456 (2004).
Firestein, G. S. Evolving concepts of rheumatoid arthritis. Nature 423, 356?361 (2003).
Keystone, E. C., Schorlemmer, H. U., Pope, C. & Allison, A. C. Zymosan-induced arthritis: a model of chronic proliferative arthritis following activation of the alternative pathway of complement. Arthritis Rheum. 20, 1396?1401 (1977).
Sakaguchi, N. et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature 426, 454?460 (2003).
Douwes, J. (1→3)-β-D-glucans and respiratory health: a review of the scientific evidence. Indoor Air 15, 160?169 (2005).
Rylander, R. & Lin, R. H. (1→3)-β-D-glucan- relationship to indoor air-related symptoms, allergy and asthma. Toxicology 152, 47?52. (2000).
Evans, S. E. et al. Pneumocystis cell wall β-glucans stimulate alveolar epithelial cell chemokine generation through nuclear factor-κB-dependent mechanisms. Am. J. Respir. Cell Mol. Biol. 32, 490?497 (2005).
Hong, F. et al. Mechanism by which orally administered β-1, 3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J. Immunol. 173, 797?806 (2004).
Rice, P. J. et al. Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J. Pharmacol. Exp. Ther. 314, 1079?1086 (2005).
Thornton, B. P., Vetvicka, V., Pitman, M., Goldman, R. C. & Ross, G. D. Analysis of the sugar specificity and molecular location of the β-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J. Immunol. 156, 1235?1246 (1996).
Xia, Y. & Ross, G. D. Generation of recombinant fragments of CD11b expressing the functional β-glucan-binding lectin site of CR3 (CD11b/CD18). J. Immunol. 162, 7285?7293 (1999).
Di Renzo, L., Yefenof, E. & Klein, E. The function of human NK cells is enhanced by β-glucan, a ligand of CR3 (CD11b/CD18). Eur. J. Immunol. 21, 1755?1758 (1991).
Ross, G. D. et al. Characterization of patients with an increased susceptibility to bacterial infections and a genetic deficiency of leukocyte membrane complement receptor type 3 and the related membrane antigen LFA-1. Blood 66, 882?890 (1985).
Tsikitis, V. L., Morin, N. A., Harrington, E. O., Albina, J. E. & Reichner, J. S. The lectin-like domain of complement receptor 3 protects endothelial barrier function from activated neutrophils. J. Immunol. 173, 1284?1291 (2004).
Xia, Y. et al. The β-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J. Immunol. 162, 2281?2290 (1999).
Hahn, P. Y. et al. Pneumocystis carinii cell wall β-glucan induces release of macrophage inflammatory protein-2 from alveolar epithelial cells via a lactosylceramide-mediated mechanism. J. Biol. Chem. 278, 2043?2050 (2003).
Wakshull, E. et al. PGG-glucan, a soluble β-(1, 3)-glucan, enhances the oxidative burst response, microbicidal activity, and activates an NF-κB-like factor in human PMN: evidence for a glycosphingolipid β-(1, 3)-glucan receptor. Immunopharmacology 41, 89?107 (1999).
Zimmerman, J. W. et al. A novel carbohydrate-glycosphingolipid interaction between a β-(1?3)-glucan immunomodulator, PGG-glucan, and lactosylceramide of human leukocytes. J. Biol. Chem. 273, 22014?22020 (1998).
Jimenez-Lucho, V., Ginsburg, V. & Krivan, H. C. Cryptococcus neoformans, Candida albicans, and other fungi bind specifically to the glycosphingolipid lactosylceramide (Gal β-1?4Glc β-1?1Cer), a possible adhesion receptor for yeasts. Infect. Immun. 58, 2085?2090 (1990).
Iwabuchi, K. & Nagaoka, I. Lactosylceramide-enriched glycosphingolipid signaling domain mediates superoxide generation from human neutrophils. Blood 100, 1454?1464 (2002).
Rice, P. J. et al. Human monocyte scavenger receptors are pattern recognition receptors for (1→3)-β-D-glucans. J. Leukoc. Biol. 72, 140?146 (2002).
Dushkin, M. I., Safina, A. F., Vereschagin, E. I. & Schwartz, Y. Carboxymethylated β-1, 3-glucan inhibits the binding and degradation of acetylated low density lipoproteins in macrophages in vitro and modulates their plasma clearance in vivo. Cell Biochem. Funct. 14, 209?217 (1996).
Vereschagin, E. I. et al. Soluble glucan protects against endotoxin shock in the rat: the role of the scavenger receptor. Shock 9, 193?198 (1998).
Pearson, A., Lux, A. & Krieger, M. Expression cloning of dSR-CI, a class C macrophage-specific scavenger receptor from Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 92, 4056?4060 (1995).
Masoud, M., Rudensky, B., Elstein, D. & Zimran, A. Chitotriosidase deficiency in survivors of Candida sepsis. Blood Cells Mol. Dis. 29, 116?118 (2002).
Yauch, L. E., Mansour, M. K., Shoham, S., Rottman, J. B. & Levitz, S. M. Involvement of CD14, toll-like receptors 2 and 4, and MyD88 in the host response to the fungal pathogen Cryptococcus neoformans in vivo. Infect. Immun. 72, 5373?5382 (2004).
Lee, S. J., Gonzalez-Aseguinolaza, G. & Nussenzweig, M. C. Disseminated candidiasis and hepatic malarial infection in mannose-binding-lectin-A-deficient mice. Mol. Cell. Biol. 22, 8199?8203 (2002).
Hogaboam, C. M., Takahashi, K., Ezekowitz, R. A., Kunkel, S. L. & Schuh, J. M. Mannose-binding lectin deficiency alters the development of fungal asthma: effects on airway response, inflammation, and cytokine profile. J. Leukoc. Biol. 75, 805?814 (2004).
Garlanda, C. et al. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature 420, 182?186 (2002).
Atochina, E. N. et al. Enhanced lung injury and delayed clearance of Pneumocystis carinii in surfactant protein A-deficient mice: attenuation of cytokine responses and reactive oxygen-nitrogen species. Infect. Immun. 72, 6002?6011 (2004).
Linke, M. J. et al. Immunosuppressed surfactant protein A-deficient mice have increased susceptibility to Pneumocystis carinii infection. J. Infect. Dis. 183, 943?952 (2001).
Atochina, E. N. et al. Delayed clearance of Pneumocystis carinii infection, increased inflammation, and altered nitric oxide metabolism in lungs of surfactant protein-D knockout mice. J. Infect. Dis. 189, 1528?1539 (2004).
Biondo, C. et al. MyD88 and TLR2, but not TLR4, are required for host defense against Cryptococcus neoformans. Eur. J. Immunol. 35, 870?878 (2005).
Netea, M. G. et al. The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J. Infect. Dis. 185, 1483?1489 (2002).
Acknowledgements
I would like to thank all my colleagues, particularly S. Gordon, for their contributions to the work on dectin-1. I thank S. Gordon, J. Willment, K. Dennehy and E. Sturrock for critically reading the manuscript. I am grateful to the Wellcome Trust and the Edward Jenner Institute for Vaccine Research for financial support. G.D.B. is a Wellcome Trust Senior Research Fellow in biomedical science in South Africa.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Supplementary information
Supplementary information S1 (movie)
S1| Phagocytosis of zymosan by cells expressing dectin-1. RAW264.7 cells (a mouse macrophage cell line), which have low levels of endogenous dectin-1 expression, were engineered to express dectin-1 and cultured in the presence of fluorescently-labelled zymosan, before being imaged by immunofluorescent microscopy. Dectin-1-expressing cells can be seen to efficiently recognize and phagocytose zymosan. The movie represents 30 minutes of real-time phagocytosis, speeded up 138-fold. Movies courtesy of S. Heinsbroek (University of Oxford). (AVI 798 kb)
Supplementary information S2 (movie)
S2| Lack of phagocytosis of zymosan by cells expressing low levels dectin-1. RAW264.7 cells (a mouse macrophage cell line), which express low levels of endogenous dectin-1 expression, were cultured in the presence of fluorescently-labelled zymosan and imaged by immunofluorescent microscopy. These cells are unable to efficiently recognize or phagocytose zymosan, unlike RAW264.7 cells engineered to express dectin-1 (see Supplementary information S1 (movie). The movie represents 30 minutes of real-time phagocytosis, speeded up 257-fold. Movies courtesy of S. Heinsbroek (University of Oxford). (AVI 1665 kb)
Glossary
- Pattern-recognition receptor
-
A receptor that binds to molecular patterns found in pathogens but not mammalian cells. Examples include dectin-1, which binds β-glucans, and Toll-like receptors, which are activated by various microbial products, such as bacterial lipopolysaccharides, hypomethylated DNA, flagellin and double-stranded RNA.
- Pathogen-associated molecular pattern
-
A molecular pattern that is found in pathogens but not mammalian cells. Examples include β-glucan, which binds dectin-1, and various microbial products, such as bacterial lipopolysaccharides, hypomethylated DNA, flagellin and double-stranded RNA, which bind Toll-like receptors.
- Opsonic recognition
-
The indirect recognition of microorganisms by specific phagocyte receptors, such as complement receptors, which recognize host serum or tissue-fluid proteins (opsonins), such as complement, that are coated (osponized) on the microbial surface.
- Respiratory burst
-
The activation of a multi-protein enzyme complex, the phagocyte nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which translocates to the phagosome membrane and generates lumenal superoxide anions through the transfer of electrons from NADPH to O2.
- Reactive oxygen intermediates
-
Toxic oxidants, such as hydrogen peroxide and hydroxyl radicals, that are produced by chemical reactions of superoxide anions in the phagosome lumen following the respiratory burst. In neutrophils, hydrogen peroxide can be further converted by myeloperoxidase into the highly toxic oxidant, hypochlorous acid.
- Type II transmembrane receptor
-
Single-pass type II transmembrane receptors have their amino (N) terminus in the cytoplasm and their carboxyl terminus on the cell surface. They have a transmembrane sequence of around 25 hydrophobic amino-acid residues but do not contain a recognizable N-terminal signal sequence, which is required for the secretion of type I receptors, which lie in the opposite orientation in the membrane.
- C-type lectin superfamily
-
A family of proteins that contain one or more C-type lectin-like domain (CTLD), which have been divided into 14 groups based on the organization of their CTLDs. The CTLDs, which were first identified as carbohydrate-recognition domains in Ca2+-dependent lectins, do not all recognize sugars, but are homologous and have a conserved sequence motif that determines the CTLD protein fold.
- Immunoreceptor tyrosine-based activation motif
-
(ITAM). A sequence that is present in the cytoplasmic domains of the invariant chains of various cell-surface immune receptors, such as the T-cell and B-cell receptor, the receptor for IgE (FcεR) and natural-killer-cell activating receptors. Following phosphorylation of their tyrosine residue, ITAMs function as docking sites for SRC homology 2 (SH2)-domain-containing tyrosine kinases and adaptor molecules, thereby facilitating intracellular-signalling cascades.
- Alternative macrophage activation
-
A state of macrophage activation, induced by the T-helper 2 (TH2) cytokines interleukin-4 (IL-4) and IL-13, that is distinct from the classical activation induced by interferon-γ, and which leads to a cellular phenotype involved in humoral immunity and repair.
- Tetraspanin
-
A family of transmembrane proteins that have four transmembrane domains and two extracellular domains of different sizes, which are defined by several conserved amino acids in the transmembrane domains. Their function is not known clearly, but they seem to interact with many other transmembrane proteins and to form large multimeric protein networks, which might be involved in intracellular signalling.
- SKG mice
-
A BALB/c-derived mouse line that spontaneously develops chronic autoimmune arthritis in non-specific-pathogen-free (non-SPF) conditions. These mice have a point mutation in a SRC homology 2 (SH2) domain of the signal transducer ζ-chain-associated protein kinase of 70 kDa in lymphocytes (ZAP70), resulting in aberrant thymic selection and the production of arthritogenic T cells. Exposure of SKG mice to environmental agents, such as β-glucans, leads to the activation of these T cells and the development of disease.
Rights and permissions
About this article
Cite this article
Brown, G. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol 6, 33–43 (2006). https://doi.org/10.1038/nri1745
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri1745