Key Points
-
Immune sensing of foreign nucleic acids among abundant self nucleic acids is a hallmark of virus detection and antiviral defence.
-
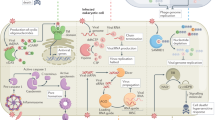
Efficient antiviral defence requires a balanced process of sensing foreign nucleic acids and ignoring self nucleic acids. This balance is accomplished by a multilevel, fail-safe system which combines immune sensing of pathogen-specific nucleic acid structures with specific labelling of self nucleic acids and nuclease-mediated degradation.
-
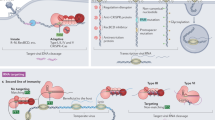
Cellular localization of nucleic acids, nucleic acid secondary structure, nucleic acid sequence and chemical modification all contribute to selective recognition of foreign nucleic acids.
-
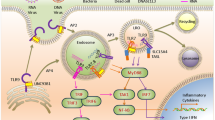
Nucleic acid sensing occurs in immune cells and non-immune cells and results in antiviral responses that include the induction of antiviral effector proteins, the secretion of cytokines alarming neighbouring cells, the secretion of chemokines, which attract immune cells, and the induction of cell death.
-
Vertebrate cells cannot completely avoid the occurrence of endogenous self nucleic acid structures with immunostimulatory properties. Therefore, additional mechanisms involving self-nucleic acid modification and nuclease-mediated degradation are necessary to diminish uncontrolled immune activation.
-
Viruses have established sophisticated mechanisms to exploit and adopt endogenous tolerance mechanisms or to avoid the presentation of characteristic molecular features recognized by nucleic acid sensing receptors.
Abstract
Innate immunity against pathogens relies on an array of immune receptors to detect molecular patterns that are characteristic of the pathogens, including receptors that are specialized in the detection of foreign nucleic acids. In vertebrates, nucleic acid sensing is the dominant antiviral defence pathway. Stimulation of nucleic acid receptors results in antiviral immune responses with the production of type I interferon (IFN), as well as the expression of IFN-stimulated genes, which encode molecules such as cell-autonomous antiviral effector proteins. This Review summarizes the tremendous progress that has been made in understanding how this sophisticated immune sensory system discriminates self from non-self nucleic acids in order to reliably detect pathogenic viruses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Isaacs, A., Cox, R. A. & Rotem, Z. Foreign nucleic acids as the stimulus to make interferon. Lancet 2, 113–116 (1963).
Junt, T. & Barchet, W. Translating nucleic acid-sensing pathways into therapies. Nat. Rev. Immunol. 15, 529–544 (2015).
Ablasser, A., Hertrich, C., Wassermann, R. & Hornung, V. Nucleic acid driven sterile inflammation. Clin. Immunol. 147, 207–215 (2013).
Wu, J. & Chen, Z. J. Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol. 32, 461–488 (2014).
Kawasaki, T., Kawai, T. & Akira, S. Recognition of nucleic acids by pattern-recognition receptors and its relevance in autoimmunity. Immunol. Rev. 243, 61–73 (2011).
Rathinam, V. A. & Fitzgerald, K. A. Cytosolic surveillance and antiviral immunity. Curr. Opin. Virol. 1, 455–462 (2012).
Desmet, C. J. & Ishii, K. J. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat. Rev. Immunol. 12, 479–491 (2012).
Yoneyama, M. & Fujita, T. Recognition of viral nucleic acids in innate immunity. Rev. Med. Virol. 20, 4–22 (2010).
Rehwinkel, J. & Reis e Sousa, C. Targeting the viral Achilles' heel: recognition of 5′-triphosphate RNA in innate anti-viral defence. Curr. Opin. Microbiol. 16, 485–492 (2013).
van den Boorn, J. G. & Hartmann, G. Turning tumors into vaccines: co-opting the innate immune system. Immunity 39, 27–37 (2013).
Svoboda, P. Renaissance of mammalian endogenous RNAi. FEBS Lett. 588, 2550–2556 (2014).
Seo, G. J. et al. Reciprocal inhibition between intracellular antiviral signaling and the RNAi machinery in mammalian cells. Cell Host Microbe 14, 435–445 (2013). This is the first study indicating reciprocal roles of RNAi and antiviral signalling in host defence.
Sato, S. et al. The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity 42, 123–132 (2015).
Weber, M. et al. Influenza virus adaptation PB2-627K modulates nucleocapsid inhibition by the pathogen sensor RIG-I. Cell Host Microbe 17, 309–319 (2015).
McAllister, C. S., Taghavi, N. & Samuel, C. E. Protein kinase PKR amplification of interferon β induction occurs through initiation factor eIF-2α-mediated translational control. J. Biol. Chem. 287, 36384–36392 (2012).
Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001). The identification of TLR3 as a dsRNA-sensing receptor.
Diebold, S. S., Kaisho, T., Hemmi, H., Akira, S. & Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 (2004).
Heil, F. et al. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303, 1526–1529 (2004). References 17 and 18 identify TLR7 as an ssRNA receptor.
Hornung, V. et al. Sequence-specific potent induction of IFN-α by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat. Med. 11, 263–270 (2005). This study describes the sequence-dependent recognition of siRNA by TLR7 and provides an explanation for sequence-dependent off-target effects caused by TLR7 activation. It is also the first introduction to RNA backbone modifications that prevent TLR7-mediated off-target effects.
Matsumoto, M. et al. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J. Immunol. 171, 3154–3162 (2003).
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737 (2004). This paper details the discovery of the RLR family.
Ahmad, S. & Hur, S. Helicases in antiviral immunity: dual properties as sensors and effectors. Trends Biochem. Sci. 40, 576–585 (2015).
Schlee, M. Master sensors of pathogenic RNA — RIG-I like receptors. Immunobiology 218, 1322–1335 (2013).
El Maadidi, S. et al. A novel mitochondrial MAVS/caspase-8 platform links RNA virus-induced innate antiviral signaling to Bax/Bak-independent apoptosis. J. Immunol. 192, 1171–1183 (2014).
Besch, R. et al. Proapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon-independent apoptosis in human melanoma cells. J. Clin. Invest. 119, 2399–2411 (2009).
Kumar, S. et al. IPS-1 differentially induces TRAIL, BCL2, BIRC3 and PRKCE in type I interferons-dependent and -independent anticancer activity. Cell Death Dis. 6, e1758 (2015).
Glas, M. et al. Targeting the cytosolic innate immune receptors RIG-I and MDA5 effectively counteracts cancer cell heterogeneity in glioblastoma. Stem Cells 31, 1064–1074 (2013).
Chattopadhyay, S. et al. Viral apoptosis is induced by IRF-3-mediated activation of Bax. EMBO J. 29, 1762–1773 (2010).
Park, S. et al. The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity. J. Immunol. 191, 4358–4366 (2013).
Subramanian, N., Natarajan, K., Clatworthy, M. R., Wang, Z. & Germain, R. N. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell 153, 348–361 (2013).
Franchi, L. et al. Cytosolic double-stranded RNA activates the NLRP3 inflammasome via MAVS-induced membrane permeabilization and K+ efflux. J. Immunol. 193, 4214–4222 (2014).
Poeck, H. et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1β production. Nat. Immunol. 11, 63–69 (2010).
Pothlichet, J. et al. Type I IFN triggers RIG-I/TLR3/NLRP3-dependent inflammasome activation in influenza A virus infected cells. PLoS Pathog. 9, e1003256 (2013).
Levin, D. & London, I. M. Regulation of protein synthesis: activation by double-stranded RNA of a protein kinase that phosphorylates eukaryotic initiation factor 2. Proc. Natl Acad. Sci. USA 75, 1121–1125 (1978).
Daffis, S. et al. 2′-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature 468, 452–456 (2010).
Pichlmair, A. et al. IFIT1 is an antiviral protein that recognizes 5′-triphosphate RNA. Nat. Immunol. 12, 624–630 (2011). References 35 and 36 report the discovery of IFIT proteins as 5′-triphosphate RNA-binding antiviral effector molecules.
Abbas, Y. M., Pichlmair, A., Gorna, M. W., Superti-Furga, G. & Nagar, B. Structural basis for viral 5′-PPP-RNA recognition by human IFIT proteins. Nature 494, 60–64 (2013).
Habjan, M. et al. Sequestration by IFIT1 impairs translation of 2′O-unmethylated capped RNA. PLoS Pathog. 9, e1003663 (2013).
Kumar, P. et al. Inhibition of translation by IFIT family members is determined by their ability to interact selectively with the 5′-terminal regions of cap0-, cap1- and 5′ppp- mRNAs. Nucleic Acids Res. 42, 3228–3245 (2014).
Zilberstein, A., Kimchi, A., Schmidt, A. & Revel, M. Isolation of two interferon-induced translational inhibitors: a protein kinase and an oligo-isoadenylate synthetase. Proc. Natl Acad. Sci. USA 75, 4734–4738 (1978).
Hovanessian, A. G., Brown, R. E. & Kerr, I. M. Synthesis of low molecular weight inhibitor of protein synthesis with enzyme from interferon-treated cells. Nature 268, 537–540 (1977).
Zhou, A., Hassel, B. A. & Silverman, R. H. Expression cloning of 2-5A-dependent RNAase: a uniquely regulated mediator of interferon action. Cell 72, 753–765 (1993).
George, C. X., John, L. & Samuel, C. E. An RNA editor, adenosine deaminase acting on double-stranded RNA (ADAR1). J. Interferon Cytokine Res. 34, 437–446 (2014).
Gelinas, J. F., Clerzius, G., Shaw, E. & Gatignol, A. Enhancement of replication of RNA viruses by ADAR1 via RNA editing and inhibition of RNA-activated protein kinase. J. Virol. 85, 8460–8466 (2011).
Weber, F., Wagner, V., Rasmussen, S. B., Hartmann, R. & Paludan, S. R. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 80, 5059–5064 (2006).
Gitlin, L. et al. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl Acad. Sci. USA 103, 8459–8464 (2006).
Kato, H. et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 (2006).
Kato, H. et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 205, 1601–1610 (2008).
Goubau, D. et al. Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5′-diphosphates. Nature 514, 372–375 (2014).
Leonard, J. N. et al. The TLR3 signaling complex forms by cooperative receptor dimerization. Proc. Natl Acad. Sci. USA 105, 258–263 (2008).
Kleinman, M. E. et al. Short-interfering RNAs induce retinal degeneration via TLR3 and IRF3. Mol. Ther. 20, 101–108 (2012).
Weber, C. et al. Toll-like receptor (TLR) 3 immune modulation by unformulated small interfering RNA or DNA and the role of CD14 (in TLR-mediated effects). Immunology 136, 64–77 (2012).
Tatematsu, M., Nishikawa, F., Seya, T. & Matsumoto, M. Toll-like receptor 3 recognizes incomplete stem structures in single-stranded viral RNA. Nat. Commun. 4, 1833 (2013).
Wu, B. et al. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell 152, 276–289 (2013).
Liddicoat, B. J. et al. RNA editing by ADAR1 prevents MDA5 sensing of endogenous dsRNA as nonself. Science 349, 1115–1120 (2015).
Pestal, K. et al. Isoforms of RNA-editing enzyme ADAR1 independently control nucleic acid sensor MDA5-driven autoimmunity and multi-organ development. Immunity 43, 933–944 (2015).
Luthra, P., Sun, D., Silverman, R. H. & He, B. Activation of IFN-β; expression by a viral mRNA through RNase L and MDA5. Proc. Natl Acad. Sci. USA 108, 2118–2123 (2011).
Runge, S. et al. In vivo ligands of MDA5 and RIG-I in measles virus-infected cells. PLoS Pathog. 10, e1004081 (2014).
Childs, K. et al. MDA-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology 359, 190–200 (2007).
Benitez, A. A. et al. In vivo RNAi screening identifies MDA5 as a significant contributor to the cellular defense against influenza A virus. Cell Rep. 11, 1714–1726 (2015).
Binder, M. et al. Molecular mechanism of signal perception and integration by the innate immune sensor retinoic acid-inducible gene-I (RIG-I). J. Biol. Chem. 286, 27278–27287 (2011).
Minks, M. A., West, D. K., Benvin, S. & Baglioni, C. Structural requirements of double-stranded RNA for the activation of 2′,5′-oligo(A) polymerase and protein kinase of interferon-treated HeLa cells. J. Biol. Chem. 254, 10180–10183 (1979).
Manche, L., Green, S. R., Schmedt, C. & Mathews, M. B. Interactions between double-stranded RNA regulators and the protein kinase DAI. Mol. Cell. Biol. 12, 5238–5248 (1992).
Judge, A. D. et al. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 23, 457–462 (2005).
Ablasser, A. et al. Selection of molecular structure and delivery of RNA oligonucleotides to activate TLR7 versus TLR8 and to induce high amounts of IL-12p70 in primary human monocytes. J. Immunol. 182, 6824–6833 (2009).
Tanji, H. et al. Toll-like receptor 8 senses degradation products of single-stranded RNA. Nat. Struct. Mol. Biol. 22, 109–115 (2015).
Shibata, T. et al. Guanosine and its modified derivatives are endogenous ligands for TLR7. Int. Immunol. 28, 211–222 (2016).
Saito, T., Owen, D. M., Jiang, F., Marcotrigiano, J. & Gale, M. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454, 523–527 (2008).
Atkinson, N. J., Witteveldt, J., Evans, D. J. & Simmonds, P. The influence of CpG and UpA dinucleotide frequencies on RNA virus replication and characterization of the innate cellular pathways underlying virus attenuation and enhanced replication. Nucleic Acids Res. 42, 4527–4545 (2014).
Tulloch, F., Atkinson, N. J., Evans, D. J., Ryan, M. D. & Simmonds, P. RNA virus attenuation by codon pair deoptimisation is an artefact of increases in CpG/UpA dinucleotide frequencies. eLife 3, e04531 (2014).
Marques, J. T. et al. A structural basis for discriminating between self and nonself double-stranded RNAs in mammalian cells. Nat. Biotechnol. 24, 559–565 (2006).
Hornung, V. et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314, 994–997 (2006).
Schlee, M. et al. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity 31, 25–34 (2009). References 72 and 73 detail the discovery and characterization of RIG-I-recognition motifs.
Vela, A., Fedorova, O., Ding, S. C. & Pyle, A. M. The thermodynamic basis for viral RNA detection by the RIG-I innate immune sensor. J. Biol. Chem. 287, 42564–42573 (2012).
Marq, J. B., Kolakofsky, D. & Garcin, D. Unpaired 5′ ppp-nucleotides, as found in arenavirus double-stranded RNA panhandles, are not recognized by RIG-I. J. Biol. Chem. 285, 18208–18216 (2010).
Wang, Y. et al. Structural and functional insights into 5′-ppp RNA pattern recognition by the innate immune receptor RIG-I. Nat. Struct. Mol. Biol. 17, 781–787 (2010). This study describes the interaction of the RIG-I RNA-binding pocket with its ligand (5′-triphosphate dsRNA) on a molecular level and details the requirements for RIG-I ligands.
Civril, F. et al. The RIG-I ATPase domain structure reveals insights into ATP-dependent antiviral signalling. EMBO Rep. 12, 1127–1134 (2011).
Jiang, F. et al. Structural basis of RNA recognition and activation by innate immune receptor RIG-I. Nature 479, 423–427 (2011).
Luo, D. et al. Structural insights into RNA recognition by RIG-I. Cell 147, 409–422 (2011).
Kowalinski, E. et al. Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell 147, 423–435 (2011).
Kohlway, A., Luo, D., Rawling, D. C., Ding, S. C. & Pyle, A. M. Defining the functional determinants for RNA surveillance by RIG-I. EMBO Rep. 14, 772–779 (2013).
Monroe, K. M., McWhirter, S. M. & Vance, R. E. Identification of host cytosolic sensors and bacterial factors regulating the type I interferon response to Legionella pneumophila. PLoS Pathog. 5, e1000665 (2009).
Abdullah, Z. et al. RIG-I detects infection with live Listeria by sensing secreted bacterial nucleic acids. EMBO J. 31, 4153–4164 (2012).
Hagmann, C. A. et al. RIG-I detects triphosphorylated RNA of Listeria monocytogenes during infection in non-immune cells. PLoS ONE 8, e62872 (2013).
Strahle, L., Garcin, D. & Kolakofsky, D. Sendai virus defective-interfering genomes and the activation of interferon-beta. Virology 351, 101–111 (2006).
Nallagatla, S. R. et al. 5′-Triphosphate-dependent activation of PKR by RNAs with short stem-loops. Science 318, 1455–1458 (2007).
Eigenbrod, T. & Dalpke, A. H. Bacterial RNA: an underestimated stimulus for innate immune responses. J. Immunol. 195, 411–418 (2015).
Kariko, K., Buckstein, M., Ni, H. & Weissman, D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005).
Eberle, F. et al. Modifications in small interfering RNA that separate immunostimulation from RNA interference. J. Immunol. 180, 3229–3237 (2008).
Schuberth-Wagner, C. et al. A conserved histidine in the RNA sensor RIG-I controls immune tolerance to N1-2′O-methylated self RNA. Immunity 43, 41–51 (2015). The paper describes Cap1 2′- O -methylation as a marker of self RNA and as a viral escape mechanism that prevents recognition by RIG-I; it details for the first time a feature in the binding pocket of an innate immune receptor that is dispensable for receptor activation but is essential for tolerance to endogenous RNA.
Devarkar, S. C. et al. Structural basis for m7G recognition and 2′-O-methyl discrimination in capped RNAs by the innate immune receptor RIG-I. Proc. Natl Acad. Sci. USA 113, 596–601 (2016).
Zust, R. et al. Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat. Immunol. 12, 137–143 (2011).
Hyde, J. L. & Diamond, M. S. Innate immune restriction and antagonism of viral RNA lacking 2-O methylation. Virology 479–480, 66–74 (2015).
Hyde, J. L. et al. A viral RNA structural element alters host recognition of nonself RNA. Science 343, 783–787 (2014).
Habjan, M. et al. Processing of genome 5′ termini as a strategy of negative-strand RNA viruses to avoid RIG-I-dependent interferon induction. PLoS ONE 3, e2032 (2008).
Anchisi, S., Guerra, J., Mottet-Osman, G. & Garcin, D. Mismatches in the influenza A virus RNA panhandle prevent retinoic acid-inducible gene I (RIG-I) sensing by impairing RNA/RIG-I complex formation. J. Virol. 90, 586–590 (2015).
Yu, P. et al. Nucleic acid-sensing Toll-like receptors are essential for the control of endogenous retrovirus viremia and ERV-induced tumors. Immunity 37, 867–879 (2012).
Malathi, K., Dong, B., Gale, M. Jr & Silverman, R. H. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature 448, 816–819 (2007).
Cho, J. A. et al. The unfolded protein response element IRE1α senses bacterial proteins invading the ER to activate RIG-I and innate immune signaling. Cell Host Microbe 13, 558–569 (2013).
Eckard, S. C. et al. The SKIV2L RNA exosome limits activation of the RIG-I-like receptors. Nat. Immunol. 15, 839–845 (2014).
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000). This paper identifies TLR9 as a receptor for DNA.
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Fernandes-Alnemri, T., Yu, J. W., Datta, P., Wu, J. & Alnemri, E. S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009).
Burckstummer, T. et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10, 266–272 (2009).
Rathinam, V. A. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 (2010).
Kerur, N. et al. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi sarcoma-associated herpesvirus infection. Cell Host Microbe 9, 363–375 (2011).
Monroe, K. M. et al. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science 343, 428–432 (2014).
Jakobsen, M. R. et al. From the cover: IFI16 senses DNA forms of the lentiviral replication cycle and controls HIV-1 replication. Proc. Natl Acad. Sci. USA 110, E4571–E4580 (2013).
Roth, S. et al. Rad50–CARD9 interactions link cytosolic DNA sensing to IL-1β production. Nat. Immunol. 15, 538–545 (2014).
Ishii, K. J. et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat. Immunol. 7, 40–48 (2006).
Ablasser, A. et al. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat. Immunol. 10, 1065–1072 (2009).
Chiu, Y. H., Macmillan, J. B. & Chen, Z. J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138, 576–591 (2009).
Stetson, D. B. & Medzhitov, R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 24, 93–103 (2006).
Paludan, S. R. Activation and regulation of DNA-driven immune responses. Microbiol. Mol. Biol. Rev. 79, 225–241 (2015).
Ishikawa, H., Ma, Z. & Barber, G. N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792 (2009).
Zhong, B. et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29, 538–550 (2008).
Sauer, J. D. et al. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect. Immun. 79, 688–694 (2011).
Burdette, D. L. et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature 478, 515–518 (2011).
Sun, L., Wu, J., Du, F., Chen, X. & Chen, Z. J. Cyclic GMP–AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013).
Wu, J. et al. Cyclic GMP–AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339, 826–830 (2012).
Gao, P. et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP–AMP synthase. Cell 153, 1094–1107 (2013). Identification of the actual second messenger structure of cGAMP as G(2′-5′)pA(3′-5′)p.
Ablasser, A. et al. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 498, 380–384 (2013).
Civril, F. et al. Structural mechanism of cytosolic DNA sensing by cGAS. Nature 498, 332–337 (2013).
Kato, K. et al. Structural and functional analyses of DNA-sensing and immune activation by human cGAS. PLoS ONE 8, e76983 (2013).
Kranzusch, P. J., Lee, A. S., Berger, J. M. & Doudna, J. A. Structure of human cGAS reveals a conserved family of second-messenger enzymes in innate immunity. Cell Rep. 3, 1362–1368 (2013).
Gao, D. et al. Cyclic GMP–AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 341, 903–906 (2013).
Zhang, X. et al. The cytosolic DNA sensor cGAS forms an oligomeric complex with DNA and undergoes switch-like conformational changes in the activation loop. Cell Rep. 6, 421–430 (2014).
Li, X. et al. Cyclic GMP–AMP synthase is activated by double-stranded DNA-induced oligomerization. Immunity 39, 1019–1031 (2013).
Li, X. D. et al. Pivotal roles of cGAS–cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341, 1390–1394 (2013).
Lam, E., Stein, S. & Falck-Pedersen, E. Adenovirus detection by the cGAS/STING/TBK1 DNA sensing cascade. J. Virol. 88, 974–981 (2014).
Lahaye, X. et al. The capsids of HIV-1 and HIV-2 determine immune detection of the viral cDNA by the innate sensor cGAS in dendritic cells. Immunity 39, 1132–1142 (2013).
Rasaiyaah, J. et al. HIV-1 evades innate immune recognition through specific cofactor recruitment. Nature 503, 402–405 (2013).
Herzner, A. M. et al. Sequence-specific activation of the DNA sensor cGAS by Y-form DNA structures as found in primary HIV-1 cDNA. Nat. Immunol. 16, 1025–1033 (2015). The first evidence for the sequence-dependent recognition of cytosolic ssDNA by cGAS.
Unterholzner, L. et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 11, 997–1004 (2010).
Jakobsen, M. R. & Paludan, S. R. IFI16: at the interphase between innate DNA sensing and genome regulation. Cytokine Growth Factor Rev. 25, 649–655 (2014).
Dutta, D. et al. BRCA1 regulates IFI16 mediated nuclear innate sensing of herpes viral DNA and subsequent induction of the innate inflammasome and interferon-β responses. PLoS Pathog. 11, e1005030 (2015).
Ferguson, B. J., Mansur, D. S., Peters, N. E., Ren, H. & Smith, G. L. DNA-PK is a DNA sensor for IRF-3-dependent innate immunity. eLife 1, e00047 (2012).
Yoh, S. M. et al. PQBP1 Is a proximal sensor of the cGAS-dependent innate response to HIV-1. Cell 161, 1293–1305 (2015).
Jin, T. et al. Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity 36, 561–571 (2012).
Martin-Gayo, E. et al. Potent cell-intrinsic immune responses in dendritic cells facilitate HIV-1-specific T cell immunity in HIV-1 elite controllers. PLoS Pathog. 11, e1004930 (2015).
Krieg, A. M. et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374, 546–549 (1995).
Hartmann, G. & Krieg, A. M. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J. Immunol. 164, 944–953 (2000). This paper shows the identification of the human CpG recognition motif.
Coch, C. et al. Higher activation of TLR9 in plasmacytoid dendritic cells by microbial DNA compared with self-DNA based on CpG-specific recognition of phosphodiester DNA. J. Leukoc. Biol. 86, 663–670 (2009).
Rigby, R. E. et al. RNA:DNA hybrids are a novel molecular pattern sensed by TLR9. EMBO J. 33, 542–558 (2014).
Mankan, A. K. et al. Cytosolic RNA:DNA hybrids activate the cGAS–STING axis. EMBO J. 33, 2937–2946 (2014).
Gehrke, N. et al. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity 39, 482–495 (2013). The first evidence for DNA damage-induced cGAS activation by TREX1-resistant oxidized DNA.
Napirei, M. et al. Features of systemic lupus erythematosus in DNase1-deficient mice. Nat. Genet. 25, 177–181 (2000).
Lan, Y. Y., Londono, D., Bouley, R., Rooney, M. S. & Hacohen, N. DNase2a deficiency uncovers lysosomal clearance of damaged nuclear DNA via autophagy. Cell Rep. 9, 180–192 (2014).
Yoshida, H., Okabe, Y., Kawane, K., Fukuyama, H. & Nagata, S. Lethal anemia caused by interferon-β produced in mouse embryos carrying undigested DNA. Nat. Immunol. 6, 49–56 (2005).
Kawane, K. et al. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature 443, 998–1002 (2006).
Baum, R. et al. Cutting edge: AIM2 and endosomal TLRs differentially regulate arthritis and autoantibody production in DNase II-deficient mice. J. Immunol. 194, 873–877 (2015).
Jakobs, C., Perner, S. & Hornung, V. AIM2 drives joint inflammation in a self-DNA triggered model of chronic polyarthritis. PLoS ONE 10, e0131702 (2015).
Stetson, D. B., Ko, J. S., Heidmann, T. & Medzhitov, R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 134, 587–598 (2008).
Gao, D. et al. Activation of cyclic GMP–AMP synthase by self-DNA causes autoimmune diseases. Proc. Natl Acad. Sci. USA 112, E5699–E5705 (2015). This study describes the impact of cytosolic DNA receptor stimulation by endogenous DNases on immune function and disease.
Ablasser, A. et al. TREX1 deficiency triggers cell-autonomous immunity in a cGAS-dependent manner. J. Immunol. 192, 5993–5997 (2014).
Gray, E. E., Treuting, P. M., Woodward, J. J. & Stetson, D. B. Cutting edge: cGAS is required for lethal autoimmune disease in the Trex1-deficient mouse model of Aicardi–Goutieres syndrome. J. Immunol. 195, 1939–1943 (2015).
Chan, M. P. et al. DNase II-dependent DNA digestion is required for DNA sensing by TLR9. Nat. Commun. 6, 5853 (2015).
Kono, H., Kimura, Y. & Latz, E. Inflammasome activation in response to dead cells and their metabolites. Curr. Opin. Immunol. 30, 91–98 (2014).
Crow, Y. J. et al. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am. J. Med. Genet. A 167, 296–312 (2015).
Lassig, C. et al. ATP hydrolysis by the viral RNA sensor RIG-I prevents unintentional recognition of self-RNA. eLife 4, e10859 (2015).
Liu, Y. et al. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 371, 507–518 (2014).
Shigemoto, T. et al. Identification of loss of function mutations in human genes encoding RIG-I and MDA5: implications for resistance to type I diabetes. J. Biol. Chem. 284, 13348–13354 (2009).
Cen, H. et al. Association of IFIH1 rs1990760 polymorphism with susceptibility to autoimmune diseases: a meta-analysis. Autoimmunity 46, 455–462 (2013).
Smith, S. & Jefferies, C. Role of DNA/RNA sensors and contribution to autoimmunity. Cytokine Growth Factor Rev. 25, 745–757 (2014).
Li, X. D. et al. Mitochondrial antiviral signaling protein (MAVS) monitors commensal bacteria and induces an immune response that prevents experimental colitis. Proc. Natl Acad. Sci. USA 108, 17390–17395 (2011).
Wang, Y. et al. Rig-I−/− mice develop colitis associated with downregulation of Gαi2. Cell Res. 17, 858–868 (2007).
Kernbauer, E., Ding, Y. & Cadwell, K. An enteric virus can replace the beneficial function of commensal bacteria. Nature 516, 94–98 (2014).
Acknowledgements
The authors would like to thank E. Bartok for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M.S. and G.H. are supported by the Deutsche Forschungsgemeinschaft (www.dfg.de); SFB670 to M.S. and G.H.; DFG SCHL1930/1–1 to M.S.; SFB704 to G.H.; SFB832 and KFO177 to G.H. G.H. and M.S. are supported by the DFG Excellence Cluster ImmunoSensation. G.H. is supported by the German Center of Infectious Disease (DZIF). G.H. is a co-founder and shareholder of Rigontec GmbH; M.S. and G.H. are inventors on a patent covering structures described in a manuscript that is cited in this Review.
Glossary
- RNA interference
-
(RNAi). The use of double-stranded RNA molecules containing sequences that match a given gene to 'knockdown' the expression of that gene by inhibiting translation of the targeted mRNA or by directing RNA-degrading enzymes to destroy the encoded mRNA transcript.
- Cyclic GMP–AMP synthetase
-
(cGAS). A cellular protein that binds to cytosolic DNA species and generates stimulator of interferon genes (STING)-activating cyclic dinucleotides.
- Inflammasome
-
A molecular complex that upon assembly activates inflammatory caspases, which in turn promote the maturation of pro-inflammatory cytokines and induce pyroptosis.
- Cap-dependent translation
-
A mechanism for initiation of translation. It involves the binding of the eIF4F complex — which is composed of the mRNA helicase eIF4A (eukaryotic translation initiation factor 4A), eIF4E and the scaffolding protein eIF4G — to mRNA transcripts that have a 5′ 7-methylguanosine cap, resulting in recruitment of the 43S ribosome. The ribosome then scans the mRNA until it reaches the first AUG codon, and it initiates translation following the GTP-dependent release of eIF2B.
- Ribonuclease L
-
(RNaseL). A constitutively expressed, latent, nonspecific endoribonuclease that is activated by 2′-5′-oligoadenylate. Type I interferons induce RNaseL expression as part of the host antiviral response.
- A-form dsRNA
-
Double-stranded RNA (dsRNA) molecules usually assemble into A-form helices within the cell. A-form helices are right-handed with 11 base pairs per helical turn, and the bases are not completely perpendicular to the helical axis. In A-form dsRNA, the major groove is deep and narrow.
- Z-form dsRNA
-
Certain conditions (high ionic strength and/or CG-rich sequences) force the transition of A-form dsRNA helices into Z-form helices. Z-form helices are left-handed with 12.4 base pairs per helical turn. In Z-form dsRNA, the minor groove is deep and narrow.
- Panhandle structures
-
Double-stranded stem structures with bulges that are formed by the partially complementary 5′- and 3′-ends of some (-)ssRNA viruses (for example, influenza virus) and are used as a replication control platform.
- Defective interfering RNA genomes
-
Virus genome mutants that are spontaneously generated owing to erroneous replication, most probably because of suboptimal replication conditions. They have lost a crucial portion of the genome. 'Snap back' defective interfering genomes occur when the replicase transcribes one strand and (without clearing the replication complex) immediately uses this new strand as a template.
- m7G cap
-
(7-methylguanosine cap). A type of cap that is linked by a triphosphate bridge to the first transcribed nucleotide at the 5′ end of eukaryotic mRNA. Recognition of the m7G cap by the cap-binding protein eIF4E is the initiation step of cap-dependent translation.
- Cap 1
-
In addition to 7-methylguanosine, the mRNA of higher eukaryotes has a 2′-O-methylation at the penultimate nucleotide (N1). This structure is called cap 1.
- Cap-snatching mechanism
-
The process by which some viruses (for example, influenza virus) cleave a 10–14 nt fragment from the capped 5′ end of cellular mRNAs and use it as a primer (leader) for their own mRNA transcription of a cap 1 structure.
- Endogenous retroviruses
-
(ERVs). 8% of the human genomic DNA contains sequences of retroviral origin that were acquired from ancestral retroviral infections and inherited into the germline.
- Unfolded protein response
-
(UPR). A response that increases the ability of the endoplasmic reticulum to fold and translocate proteins, decreases the synthesis of proteins and causes the arrest of the cell cycle and apoptosis.
- SKIV2L RNA exosome
-
A multiprotein cellular complex in eukaryotes that degrades various types of RNA. In this way, the cytosolic 3′-to-5′ RNA exosome complex, defined by the SKIV2L RNA helicase subunit, is a crucial negative regulator that prevents the stimulation of RIG-I-like receptors by endogenous RNA.
- DNA-PK complex
-
The nuclear DNA-dependent serine/threonine protein kinase (DNA-PK) complex is composed of the proteins DNA-PKcs, Ku70 and Ku80. It is involved in DNA non-homologous end-joining during DNA double-strand break repair.
- Interferon stimulatory DNA pathway
-
(ISD pathway). A cytosolic dsDNA recognition pathway that involves the second messenger receptor STING, but not RIG-I, and leads to type I IFN production. The ISD pathway is triggered by any random dsDNA sequence of a certain minimum length (40–60 bp).
- Y-form DNA
-
DNA that contains a region of base-paired DNA at one end and a region of unpaired DNA strands at the opposite end.
- Elite controllers of HIV1 infection
-
HIV1-infected individuals with an immune system that can limit HIV1 infection without antiviral treatment.
- Autophagy
-
An evolutionarily conserved process in which acidic double-membrane vacuoles sequester intracellular contents (such as damaged organelles and macromolecules) and target them for degradation through fusion to secondary lysosomes.
- Aicardi–Goutières syndrome
-
(AGS). A neurodegenerative disorder that can be caused by stimulator of IFN genes (STING)-dependent cytokine hyperproduction owing to mutations in genes such as TREX1.
- Necroptosis
-
A type of necrosis and a form of non-apoptotic cell death driven by receptor-interacting serine/threonine-protein kinase 1 (RIPK1) and RIPK3 under conditions in which caspase 8 is inhibited.
- Pyroptosis
-
An inflammatory and lytic form of programmed cell death mediated by inflammatory caspases.
- MicroRNAs
-
Small, RNA molecules that regulate the expression of genes by binding to the 3′-untranslated regions (3′-UTR) of specific mRNAs.
Rights and permissions
About this article
Cite this article
Schlee, M., Hartmann, G. Discriminating self from non-self in nucleic acid sensing. Nat Rev Immunol 16, 566–580 (2016). https://doi.org/10.1038/nri.2016.78
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2016.78