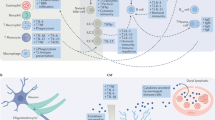
Key Points
-
Across evolutionary time, inflammatory responses and depressive symptoms were part of an integrated adaptive response to pathogens that facilitated fighting infection, healing wounds and avoiding subsequent pathogen exposure in the pathogen-rich environments in which humans evolved. In the more sanitary environments of the modern world, the relationship between inflammatory pathways and the brain may drive depression and contribute to non-response to antidepressant medication.
-
Increased levels of inflammatory cytokines and induction of their signalling pathways as well as activation of different immune cell subsets has been detected in the brain and peripheral blood of a subgroup of patients with depression. C-reactive protein (CRP), tumour necrosis factor, interleukin-1β (IL-1β) and IL-6 appear to be the most reliably elevated inflammatory markers in the peripheral blood of subjects with depression.
-
Activation of the inflammasome by stress-induced, non-pathogenic stimuli, including damage-associated molecular patterns as well as microbial-associated molecular patterns elaborated from the gut microbiome, may drive peripheral inflammatory responses, which are then transmitted to the brain by trafficking of activated monocytes.
-
Inflammation impacts several neurotransmitter systems in the brain, including serotonin, dopamine and glutamate pathways, as well as the kynurenine pathway, which generates the neurotoxic metabolite quinolinic acid. Neuroimaging studies have demonstrated that disruption of neurotransmitter pathways is associated with inflammation-induced alterations in brain circuits that mediate motivation and motor activity as well as anxiety, arousal and alarm.
-
Activation of effector T cells during stress can prevent the development of depressive- and anxiety-like behaviour in mice. These effects may be mediated by the trafficking of effector T cells to the meningeal space where they produce IL-4, which supports anti-inflammatory responses while also stimulating the production of growth factors in the brain that support neural plasticity and resilience.
-
Studies in depression suggest that inflammatory biomarkers, such as CRP, can be used to enrich samples for anti-inflammatory clinical trials for depression that target inflammation-related symptoms such as anhedonia and anxiety, thereby supporting intelligent trial design. Though still in development, imaging of neuroinflammation will help establish a 'target' in the brain to further facilitate the testing of anti-inflammatory therapies for depression.
Abstract
Crosstalk between inflammatory pathways and neurocircuits in the brain can lead to behavioural responses, such as avoidance and alarm, that are likely to have provided early humans with an evolutionary advantage in their interactions with pathogens and predators. However, in modern times, such interactions between inflammation and the brain appear to drive the development of depression and may contribute to non-responsiveness to current antidepressant therapies. Recent data have elucidated the mechanisms by which the innate and adaptive immune systems interact with neurotransmitters and neurocircuits to influence the risk for depression. Here, we detail our current understanding of these pathways and discuss the therapeutic potential of targeting the immune system to treat depression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386, 743–800 (2015).
Rush, A. J. et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry 163, 1905–1917 (2006).
Pace, T. W. et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry 163, 1630–1633 (2006).
Bierhaus, A. et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl Acad. Sci. USA 100, 1920–1925 (2003). This study is one of the first demonstrations that a psychological stressor could activate fundamental inflammatory signalling pathways (that is, NF-κB) in human peripheral blood mononuclear cells.
Aschbacher, K. et al. Maintenance of a positive outlook during acute stress protects against pro-inflammatory reactivity and future depressive symptoms. Brain Behav. Immun. 26, 346–352 (2012).
Raison, C. L. & Miller, A. H. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D). Mol. Psychiatry 18, 15–37 (2013). This theoretical treatise proposes that depression, rather than being a maladaptive response to psychosocial challenge, is the outgrowth of an evolutionary advantage provided by a crosstalk between the immune system and the brain to survive ancestral challenges from pathogens and predators.
Watson, P. J. & Andrews, P. W. Toward a revised evolutionary adaptationist analysis of depression: the social navigation hypothesis. J. Affect. Disord. 72, 1–14 (2002).
Kinney, D. K. & Tanaka, M. An evolutionary hypothesis of depression and its symptoms, adaptive value, and risk factors. J. Nerv. Ment. Dis. 197, 561–567 (2009).
Slavich, G. M. & Irwin, M. R. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol. Bull. 140, 774–815 (2014).
Seedat, S. et al. Cross-national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. Arch. Gen. Psychiatry 66, 785–795 (2009).
Moieni, M. et al. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology 40, 1709–1716 (2015).
Udina, M. et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J. Clin. Psychiatry 73, 1128–1138 (2012).
Raison, C. L., Lowry, C. A. & Rook, G. A. Inflammation, sanitation, and consternation: loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch. Gen. Psychiatry 67, 1211–1224 (2010).
Rook, G. A., Lowry, C. A. & Raison, C. L. Hygiene and other early childhood influences on the subsequent function of the immune system. Brain Res. 1617, 47–62 (2015).
Yirmiya, R. et al. Illness, cytokines, and depression. Ann. NY Acad. Sci. 917, 478–487 (2000).
Miller, A. H., Maletic, V. & Raison, C. L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65, 732–741 (2009).
Maes, M. Major depression and activation of the inflammatory response system. Adv. Exp. Med. Biol. 461, 25–46 (1999).
Brambilla, P. et al. Increased M1/decreased M2 signature and signs of Th1/Th2 shift in chronic patients with bipolar disorder, but not in those with schizophrenia. Transl Psychiatry 4, e406 (2014).
Drago, A., Crisafulli, C., Calabro, M. & Serretti, A. Enrichment pathway analysis. The inflammatory genetic background in bipolar disorder. J. Affect Disord. 179, 88–94 (2015).
Mostafavi, S. et al. Type I interferon signaling genes in recurrent major depression: increased expression detected by whole-blood RNA sequencing. Mol. Psychiatry 19, 1267–1274 (2013).
Maes, M. Evidence for an immune response in major depression: a review and hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry 19, 11–38 (1995). In contrast to previous theories that primarily focused on reduced T cell responses to mitogenic stimuli, this is one of the first papers to posit that an activated immune system may have a role in the aetiology of depression.
Bufalino, C., Hepgul, N., Aguglia, E. & Pariante, C. M. The role of immune genes in the association between depression and inflammation: a review of recent clinical studies. Brain Behav. Immun. 31, 31–47 (2012).
Capuron, L. et al. Neurobehavioral effects of interferon-α in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology 26, 643–652 (2002).
Reichenberg, A. et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatry 58, 445–452 (2001).
Bonaccorso, S. et al. Increased depressive ratings in patients with hepatitis C receiving interferon-α-based immunotherapy are related to interferon-α-induced changes in the serotonergic system. J. Clin. Psychopharmacol. 22, 86–90 (2002).
Harrison, N. A. et al. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatry 66, 407–414 (2009).
Tyring, S. et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet 367, 29–35 (2006).
Abbott, R. et al. Tumour necrosis factor-α inhibitor therapy in chronic physical illness: a systematic review and meta-analysis of the effect on depression and anxiety. J. Psychosom. Res. 79, 175–84 (2015).
Kohler, O. et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 71, 1381–1391 (2014).
Raison, C. L. et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 70, 31–41 (2013). This report describes the results of the first double-blind, placebo-controlled trial of a monoclonal antibody against TNF to treat major depression, indicating that only patients with depression that have high levels of inflammation respond to cytokine antagonism.
Miller, A. H. & Raison, C. L. Are anti-inflammatory therapies viable treatments for psychiatric disorders?: where the rubber meets the road. JAMA Psychiatry 72, 527–528 (2015).
Michopoulos, V. et al. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am. J. Psychiatry 172, 353–362 (2015).
Fernandes, B. S. et al. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol. Psychiatry http://dx.doi.org/10.1038/mp.2015.87 (2015).
Morris, S. E. & Cuthbert, B. N. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin. Neurosci. 14, 29–37 (2012).
Miller, A. H., Haroon, E., Raison, C. L. & Felger, J. C. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress. Anxiety 30, 297–306 (2013).
Cattaneo, A. et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline 'predictors' and longitudinal 'targets'. Neuropsychopharmacology 38, 377–385 (2013).
Eurelings, L. S., Richard, E., Eikelenboom, P., van Gool, W. A. & Moll van Charante, E. P. Low-grade inflammation differentiates between symptoms of apathy and depression in community-dwelling older individuals. Int. Psychogeriatr. 27, 639–647 (2015).
Pearson, T. A. et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107, 499–511 (2003).
Irwin, M. R. & Cole, S. W. Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol. 11, 625–632 (2011).
Iwata, M., Ota, K. T. & Duman, R. S. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav. Immun. 31, 105–114 (2013).
Strowig, T., Henao-Mejia, J., Elinav, E. & Flavell, R. Inflammasomes in health and disease. Nature 481, 278–286 (2012).
Fleshner, M. Stress-evoked sterile inflammation, danger associated molecular patterns (DAMPs), microbial associated molecular patterns (MAMPs) and the inflammasome. Brain Behav. Immun. 27, 1–7 (2013).
Cox, S. S. et al. Adrenergic and glucocorticoid modulation of the sterile inflammatory response. Brain Behav. Immun. 36, 183–192 (2014).
Pan, Y., Chen, X. Y., Zhang, Q. Y. & Kong, L. D. Microglial NLRP3 inflammasome activation mediates IL-1β-related inflammation in prefrontal cortex of depressive rats. Brain Behav. Immun. 41, 90–100 (2014).
Zhang, Y. et al. NLRP3 inflammasome mediates chronic mild stress-induced depression in mice via neuroinflammation. Int. J. Neuropsychopharmacol. 18, pii: pyv006 (2015).
Paugh, S. W. et al. NALP3 inflammasome upregulation and CASP1 cleavage of the glucocorticoid receptor cause glucocorticoid resistance in leukemia cells. Nat. Genet. 47, 607–614 (2015).
Rhen, T. & Cidlowski, J. A. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N. Engl. J. Med. 353, 1711–1723 (2005).
Raison, C. L. & Miller, A. H. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am. J. Psychiatry 160, 1554–1565 (2003).
Pace, T. W., Hu, F. & Miller, A. H. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav. Immun. 21, 9–19 (2007).
Alcocer-Gomez, E. et al. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav. Immun. 36, 111–117 (2014). This paper provides the first indication that activation of the inflammasome may contribute to elevated levels of inflammatory cytokines such as IL-1β and IL-18 in major depression, consistent with studies in laboratory animal models of depression that demonstrated that inhibition of NLRP3 can block the development of stress-induced depressive-like behaviour.
Stertz, L. et al. Damage-associated molecular patterns and immune activation in bipolar disorder. Acta Psychiatr. Scand. 132, 211–217 (2015).
Rawdin, B. J. et al. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav. Immun. 31, 143–152 (2013).
Maes, M., Galecki, P., Chang, Y. S. & Berk, M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 676–692 (2011).
Mayer, E. A., Knight, R., Mazmanian, S. K., Cryan, J. F. & Tillisch, K. Gut microbes and the brain: paradigm shift in neuroscience. J. Neurosci. 34, 15490–15496 (2014).
Maslanik, T. et al. Commensal bacteria and MAMPs are necessary for stress-induced increases in IL-1β and IL-18 but not IL-6, IL-10 or MCP-1. PLoS ONE 7, e50636 (2012).
Lyte, M., Vulchanova, L. & Brown, D. R. Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell Tissue Res. 343, 23–32 (2011).
Rao, J. S., Harry, G. J., Rapoport, S. I. & Kim, H. W. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol. Psychiatry 15, 384–392 (2010).
Steiner, J. et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J. Psychiatr. Res. 42, 151–157 (2008).
Torres-Platas, S. G., Cruceanu, C., Chen, G. G., Turecki, G. & Mechawar, N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav. Immun. 42, 50–59 (2014). This study provides some of the most compelling evidence that neuroinflammation occurs in major depression by demonstrating that monocytes traffic to the brain of patients with depressive symptoms and assume a perivascular localization in association with chemoattractant molecules such as CCL2, which has been shown to attract monocytes to the brain in animal models of stress.
Nagy, C. et al. Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol. Psychiatry 20, 320–328 (2015).
Setiawan, E. et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72, 268–275 (2015).
Hannestad, J. et al. The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: a [11C]PBR28 PET study. Brain Behav. Immun. 33, 131–138 (2013).
Sandiego, C. M. et al. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc. Natl Acad. Sci. USA 112, 12468–12473 (2015).
Quan, N. & Banks, W. A. Brain-immune communication pathways. Brain Behav. Immun. 21, 727–735 (2007).
D'Mello, C., Le, T. & Swain, M. G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factor-α signaling during peripheral organ inflammation. J. Neurosci. 29, 2089–2102 (2009).
Hennessy, E., Griffin, E. W. & Cunningham, C. Astrocytes are primed by chronic neurodegeneration to produce exaggerated chemokine and cell infiltration responses to acute stimulation with the cytokines IL-1β and TNF-α. J. Neurosci. 35, 8411–8422 (2015).
Wohleb, E. S. et al. β-adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J. Neurosci. 31, 6277–6288 (2011).
Wohleb, E. S., Powell, N. D., Godbout, J. P. & Sheridan, J. F. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J. Neurosci. 33, 13820–13833 (2013). References 67 and 68 present a series of experiments that demonstrate a cellular pathway by which cytokines signals can be transmitted to the brain via trafficking of monocytes from the bone marrow to the brain parenchyma, a process mediated by catecholamines and CCL2.
Krugel, U., Fischer, J., Radicke, S., Sack, U. & Himmerich, H. Antidepressant effects of TNF-α blockade in an animal model of depression. J. Psychiatr. Res. 47, 611–616 (2013).
Arends, S. et al. Baseline predictors of response and discontinuation of tumor necrosis factor-α blocking therapy in ankylosing spondylitis: a prospective longitudinal observational cohort study. Arthritis Res. Ther. 13, R94 (2011).
Gillespie, C. F., Garlow, S. J., Binder, E. B., Schatzberg, A. F. & Nemeroff, C. B. in Textbook of Psychopharmacology (eds Schatzberg, A. F. & Nemeroff, C. B.) 903–944 (America Psychiatric Publishing, 2009).
Zhu, C. B. et al. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology 35, 2510–2520 (2010).
Neurauter, G. et al. Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr. Drug Metab. 9, 622–627 (2008).
Felger, J. C. et al. Tyrosine metabolism during interferon-α administration: association with fatigue and CSF dopamine concentrations. Brain Behav. Immun. 31, 153–160 (2013).
Maes, M., Leonard, B. E., Myint, A. M., Kubera, M. & Verkerk, R. The new '5-HT' hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 702–721 (2011).
Raison, C. L. et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-α: relationship to CNS immune responses and depression. Mol. Psychiatry 15, 393–403 (2010).
Steiner, J. et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J. Neuroinflamm. 8, 94 (2011).
Tavares, R. G. et al. Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochem. Int. 40, 621–627 (2002).
Tilleux, S. & Hermans, E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J. Neurosci. Res. 85, 2059–2070 (2007).
Hardingham, G. E., Fukunaga, Y. & Bading, H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 5, 405–414 (2002).
Koo, J. W., Russo, S. J., Ferguson, D., Nestler, N. J. & Duman, R. S. Nuclear factor-κB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl Acad. Sci. USA 107, 2669–2674 (2010).
Goshen, I. et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol. Psychiatry 13, 717–728 (2008).
Haroon, E. et al. IFN-α-induced cortical and subcortical glutamate changes assessed by magnetic resonance spectroscopy. Neuropsychopharmacology 39, 1777–1785 (2014).
Haroon, E. et al. Conceptual convergence:increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol. Psychiatry http://dx.doi.org/10.1038/mp.2015.206 (2015).
Walker, A. K. et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 38, 1609–1616 (2013).
O'Connor, J. C. et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 14, 511–522 (2009).
Duman, R. S. & Monteggia, L. M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 59, 1116–1127 (2006).
Hodes, G. E. et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl Acad. Sci. USA 111, 16136–16141 (2014). This paper gives compelling evidence in laboratory animals that individual differences in the behavioural response to a social stressor is mediated by individual differences in the production of the inflammatory cytokine IL-6, suggesting that genetic and/or environmental factors that regulate inflammatory responses can determine stress-induced depressive-like behaviour.
Capuron, L. et al. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon-α administration. Arch. Gen. Psychiatry 69, 1044–1053 (2012). Using multimodal neuroimaging, this report provides an integration of the impact of inflammatory cytokines on reward pathways and dopamine metabolism in the basal ganglia that leads to alterations in behaviours relevant to motivation and ultimately anhedonia — a core symptom of depression.
Eisenberger, N. I. et al. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol. Psychiatry 68, 748–754 (2010).
Felger, J. C. et al. Chronic interferon-α decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology 38, 2179–2187 (2013).
Dowell, N. G. et al. Acute changes in striatal microstructure predict the development of interferon-α induced fatigue. Biol. Psychiatry http://dx.doi.org/10.1016/j.biopsych.2015.05.015 (2015).
Harrison, N. A., Cercignani, M., Voon, V. & Critchley, H. D. Effects of inflammation on hippocampus and substantia nigra responses to novelty in healthy human participants. Neuropsychopharmacology 40, 831–838 (2015).
Harrison, N. A. et al. A neuro-computational account of how inflammation enhances sensitivity to punishments versus rewards. Biol. Psychiatry http://dx.doi.org/10.1016/j.biopsych.2015.07.018 (2015).
Felger, J. C. et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry http://dx.doi.org/10.1038/mp.2015.168 (2015). This neuroimaging study is the first to demonstrate that the impact of immune stimuli (for example, IFN α, typhoid vaccination and endotoxin) on the brains of otherwise non-depressed individuals extended to patients with depression, whose increased inflammation was found to reduce functional connectivity in reward-related neurocircuitry leading to anhedonia, a core symptom of depression.
Harrison, N. A. et al. Neural origins of human sickness in interoceptive responses to inflammation. Biol. Psychiatry 66, 415–422 (2009).
Slavich, G. M., Way, B. M., Eisenberger, N. I. & Taylor, S. E. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc. Natl Acad. Sci. USA 107, 14817–14822 (2010). This manuscript provides one of the first and most detailed descriptions of the relationship between stress-induced activation of inflammatory responses and sensitivity to a psychosocial stressor, identifying key brain regions such as the dACC as a target of cytokines, leading to anxiety, arousal and alarm.
Eisenberger, N. I. & Lieberman, M. D. Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn. Sci. 8, 294–300 (2004).
Muscatell, K. A. et al. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav. Immun. 43, 46–53 (2015).
Gimeno, D. et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol. Med. 39, 413–423 (2009).
Au, B., Smith, K. J., Gariepy, G. & Schmitz, N. The longitudinal associations between C-reactive protein and depressive symptoms: evidence from the English Longitudinal Study of Ageing (ELSA). Int. J. Geriatr. Psychiatry 30, 976–984 (2015).
Duivis, H. E. et al. Depressive symptoms, health behaviors, and subsequent inflammation in patients with coronary heart disease: prospective findings from the heart and soul study. Am. J. Psychiatry 168, 913–920 (2011).
Baumeister, D., Akhtar, R., Ciufolini, S., Pariante, C. M. & Mondelli, V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol. Psychiatry http://dx.doi.org/10.1038/mp.2015.67 (2015).
Danese, A., Pariante, C. M., Caspi, A., Taylor, A. & Poulton, R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl Acad. Sci. USA 104, 1319–1324 (2007).
Tartter, M., Hammen, C., Bower, J. E., Brennan, P. A. & Cole, S. Effects of chronic interpersonal stress exposure on depressive symptoms are moderated by genetic variation at IL6 and IL1β in youth. Brain Behav. Immun. 46, 104–111 (2015).
Klengel, T. et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 16, 33–41 (2013).
Miller, G. E. et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-κB signaling. Biol. Psychiatry 64, 266–272 (2008).
Brachman, R. A., Lehmann, M. L., Maric, D. & Herkenham, M. Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. J. Neurosci. 35, 1530–1538 (2015).
Lewitus, G. M., Cohen, H. & Schwartz, M. Reducing post-traumatic anxiety by immunization. Brain Behav. Immun. 22, 1108–1114 (2008).
Lewitus, G. M. et al. Vaccination as a novel approach for treating depressive behavior. Biol. Psychiatry 65, 283–288 (2009). References 109 and 110 provide the first evidence that engaging T cells to traffic to the brain during stress can block depressive- and anxiety-like behaviour and induce growth factors and neurogenesis in the brain, representing an entirely novel approach to the treatment of depression that takes advantage of the capacity of T cells to support neuronal integrity.
Derecki, N. C. et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med. 207, 1067–1080 (2010).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Kim, S. J. et al. CD4+CD25+ regulatory T cell depletion modulates anxiety and depression-like behaviors in mice. PLoS ONE 7, e42054 (2012).
Rosas-Ballina, M. et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101 (2011).
Bauer, M. E. et al. Dexamethasone-induced effects on lymphocyte distribution and expression of adhesion molecules in treatment-resistant depression. Psychiatry Res. 113, 1–15 (2002).
Wang, X., Wu, H. & Miller, A. H. Interleukin 1α (IL-1α) induced activation of p38 mitogen-activated protein kinase inhibits glucocorticoid receptor function. Mol. Psychiatry 9, 65–75 (2004).
Wei, J., Zhang, M. & Zhou, J. Myeloid-derived suppressor cells in major depression patients suppress T-cell responses through the production of reactive oxygen species. Psychiatry Res. 228, 695–701 (2015).
van Deventer, H. W. et al. The inflammasome component NLRP3 impairs antitumor vaccine by enhancing the accumulation of tumor-associated myeloid-derived suppressor cells. Cancer Res. 70, 10161–10169 (2010).
Li, Y. et al. Altered expression of CD4+CD25+ regulatory T cells and its 5-HT1a receptor in patients with major depression disorder. J. Affect. Disord. 124, 68–75 (2010).
Williamson, L. L. et al. Got worms? Perinatal exposure to helminths prevents persistent immune sensitization and cognitive dysfunction induced by early-life infection. Brain Behav. Immun. (2015).
Olofsson, P. S., Rosas-Ballina, M., Levine, Y. A. & Tracey, K. J. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol. Rev. 248, 188–204 (2012).
Wang, Y., Chen, X., Cao, W. & Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat. Immunol. 15, 1009–1016 (2014).
Warner-Schmidt, J. L., Vanover, K. E., Chen, E. Y., Marshall, J. J. & Greengard, P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc. Natl Acad. Sci. USA 108, 9262–9267 (2011).
Gurven, M. & Kaplan, H. Longevity among hunter-gatherers: a cross-cultural examination. Popul. Dev. Rev. 33, 321–365 (2007).
Fumagalli, M. et al. Signatures of environmental genetic adaptation pinpoint pathogens as the main selective pressure through human evolution. PLoS Genet. 7, e1002355 (2011).
Kuningas, M. et al. Selection for genetic variation inducing pro-inflammatory responses under adverse environmental conditions in a Ghanaian population. PLoS ONE 4, e7795 (2009).
Ratcliffe, M. A bad case of the flu?: the comparative phenomenology of depression and somatic illness. J. Conscious. Studies 20, 198–218 (2013).
Marin, I. & Kipnis, J. Learning and memory... and the immune system. Learn. Mem. 20, 601–606 (2013).
Mehta, D. et al. Transcriptional signatures related to glucose and lipid metabolism predict treatment response to the tumor necrosis factor antagonist infliximab in patients with treatment-resistant depression. Brain Behav. Immun. 31, 205–215 (2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Conspecifics
-
Members of the same species.
- Sickness behaviour
-
An adaptive response to illness, often precipitated by infection, that includes social withdrawal, decreased appetite, lethargy, impaired concentration, depressed mood, irritability, muscle aches and pain, and fever. This syndrome is believed to prioritize shifting of energy resources to fighting infection and wound healing.
- Anhedonia
-
A lack of interest in usually pleasurable activities that represents a decrease in motivation, which can either represent a decrease in the response to reward or in the willingness to expend effort to obtain reward.
- Major depressive disorder
-
A clinical syndrome of depression characterized by the primary symptoms of depressed mood and anhedonia, and diagnosed using criteria set forth by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.
- Social defeat stress
-
A model of depression that entails repeated exposure to a conspecific animal screened for aggressive behaviour. The animals are placed together in the same cage where they are exposed to brief bouts of defeat lasting 5–10 minutes daily typically for 6–10 days.
- Myeloid-derived suppressor cells
-
A heterogeneous population of cells of myeloid origin that rapidly expands during inflammation and can potently suppress T cell responses. They are now being explored as potential therapeutic targets to inhibit immune responses in autoimmune disease or transplant rejection.
- Cytokine hypothesis of depression
-
A theoretical framework that suggests that cytokines have a primary role in alterations of neurotransmitter metabolism, neuroendocrine function, neuroplasticity, neurocircuitry and behaviour in a subgroup of patients with depression and increased inflammation.
Rights and permissions
About this article
Cite this article
Miller, A., Raison, C. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16, 22–34 (2016). https://doi.org/10.1038/nri.2015.5
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2015.5
This article is cited by
-
Dose-response associations of maternal prenatal noise exposure duration with antepartum depression status
BMC Pregnancy and Childbirth (2024)
-
The quest for a biological phenotype of adolescent non-suicidal self-injury: a machine-learning approach
Translational Psychiatry (2024)
-
Quantitative MRI at 7-Tesla reveals novel frontocortical myeloarchitecture anomalies in major depressive disorder
Translational Psychiatry (2024)
-
Evaluation of serum interleukin-12 and interleukin-4 as potential biomarkers for the diagnosis of major depressive disorder
Scientific Reports (2024)
-
Elevated body temperature is associated with depressive symptoms: results from the TemPredict Study
Scientific Reports (2024)