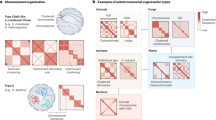
Key Points
-
CCCTC-binding factor (CTCF) is an architectural protein that can mediate both interchromosomal and intrachromosomal interactions.
-
The functional outcomes of these interactions depend on the nature of the sequences adjacent to CTCF-binding sites and perhaps on the presence of other chromatin proteins.
-
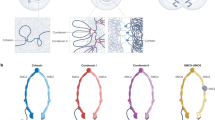
CTCF-mediated chromatin loops regulate diverse nuclear processes, including V(D)J recombination, enhancer–promoter interactions, transcriptional pausing and alternative mRNA splicing.
-
The consensus sequence of CTCF-binding sites is highly conserved. Its variable DNA occupancy pattern is regulated by DNA methylation, non-coding RNAs and post-translational modification.
-
CTCF and other architectural proteins, such as cohesin and TFIIIC, maintain genome organization by clustering at the boundaries of megabase-scale topologically associating domains.
-
Cell-type-specific chromatin organization occurs at the sub-megabase scale; CTCF, either alone or in combination with other proteins, regulates specific transcriptional processes.
Abstract
The eukaryotic genome is organized in the three-dimensional nuclear space in a specific manner that is both a cause and a consequence of its function. This organization is partly established by a special class of architectural proteins, of which CCCTC-binding factor (CTCF) is the best characterized. Although CTCF has been assigned various roles that are often contradictory, new results now help to draw a unifying model to explain the many functions of this protein. CTCF creates boundaries between topologically associating domains in chromosomes and, within these domains, facilitates interactions between transcription regulatory sequences. Thus, CTCF links the architecture of the genome to its function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Van Bortle, K. & Corces, V. G. Nuclear organization and genome function. Annu. Rev. Cell Dev. Biol. 28, 163–187 (2012).
de Laat, W. & Dekker, J. 3C-based technologies to study the shape of the genome. Methods 58, 189–191 (2012).
Baniahmad, A., Steiner, C., Kohne, A. C. & Renkawitz, R. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell 61, 505–514 (1990).
Lobanenkov, V. V. et al. A novel sequence-specific DNA binding protein which interacts with three regularly spaced direct repeats of the CCCTC-motif in the 5′-flanking sequence of the chicken c-myc gene. Oncogene 5, 1743–1753 (1990).
Heger, P., Marin, B., Bartkuhn, M., Schierenberg, E. & Wiehe, T. The chromatin insulator CTCF and the emergence of metazoan diversity. Proc. Natl Acad. Sci. USA 109, 17507–17512 (2012).
Ohlsson, R., Renkawitz, R. & Lobanenkov, V. CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends Genet. 17, 520–527 (2001).
Chen, H., Tian, Y., Shu, W., Bo, X. & Wang, S. Comprehensive identification and annotation of cell type-specific and ubiquitous CTCF-binding sites in the human genome. PLoS ONE 7, e41374 (2012).
Cuddapah, S. et al. Global analysis of the insulator binding protein CTCF in chromatin barrier regions reveals demarcation of active and repressive domains. Genome Res. 19, 24–32 (2009).
Schmidt, D. et al. Waves of retrotransposon expansion remodel genome organization and CTCF binding in multiple mammalian lineages. Cell 148, 335–348 (2012).
Barski, A. et al. High-resolution profiling of histone methylations in the human genome. Cell 129, 823–837 (2007).
Kim, T. H. et al. Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell 128, 1231–1245 (2007).
Chen, X. et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133, 1106–1117 (2008).
DeMare, L. E. et al. The genomic landscape of cohesin-associated chromatin interactions. Genome Res. 23, 1224–1234 (2013).
Song, L. et al. Open chromatin defined by DNaseI and FAIRE identifies regulatory elements that shape cell-type identity. Genome Res. 21, 1757–1767 (2011).
Rhee, H. S. & Pugh, B. F. Comprehensive genome-wide protein–DNA interactions detected at single-nucleotide resolution. Cell 147, 1408–1419 (2011).
Nakahashi, H. et al. A genome-wide map of CTCF multivalency redefines the CTCF code. Cell Rep. 3, 1678–1689 (2013). In this study, genome-wide mapping of different CTCF mutants reveals that combinatorial clustering of its 11 zinc-fingers recognizes a wide range of DNA modules.
Engel, N., West, A. G., Felsenfeld, G. & Bartolomei, M. S. Antagonism between DNA hypermethylation and enhancer-blocking activity at the H19 DMD is uncovered by CpG mutations. Nature Genet. 36, 883–888 (2004).
Rodriguez, C. et al. CTCF is a DNA methylation-sensitive positive regulator of the INK/ARF locus. Biochem. Biophys. Res. Commun. 392, 129–134 (2010).
Lai, A. Y. et al. DNA methylation prevents CTCF-mediated silencing of the oncogene BCL6 in B cell lymphomas. J. Exp. Med. 207, 1939–1950 (2010).
Chang, J. et al. Nicotinamide adenine dinucleotide (NAD)-regulated DNA methylation alters CCCTC-binding factor (CTCF)/cohesin binding and transcription at the BDNF locus. Proc. Natl Acad. Sci. USA 107, 21836–21841 (2010).
Wang, H. et al. Widespread plasticity in CTCF occupancy linked to DNA methylation. Genome Res. 22, 1680–1688 (2012). This paper shows that cell-type-specific CTCF-binding sites across 19 diverse human cell types are linked to the differential DNA methylation patterns within the cells.
Zampieri, M. et al. ADP-ribose polymers localized on Ctcf–Parp1–Dnmt1 complex prevent methylation of Ctcf target sites. Biochem. J. 441, 645–652 (2012).
Guastafierro, T. et al. CCCTC-binding factor activates PARP-1 affecting DNA methylation machinery. J. Biol. Chem. 283, 21873–21880 (2008).
Kriaucionis, S. & Heintz, N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 324, 929–930 (2009).
Tahiliani, M. et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324, 930–935 (2009).
Ito, S. et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 333, 1300–1303 (2011).
Yu, M. et al. Base-resolution analysis of 5-hydroxymethylcytosine in the mammalian genome. Cell 149, 1368–1380 (2012).
Song, C. X. et al. Genome-wide profiling of 5-formylcytosine reveals its roles in epigenetic priming. Cell 153, 678–691 (2013).
Spruijt, C. G. et al. Dynamic readers for 5-(hydroxy)methylcytosine and its oxidized derivatives. Cell 152, 1146–1159 (2013).
MacPherson, M. J., Beatty, L. G., Zhou, W., Du, M. & Sadowski, P. D. The CTCF insulator protein is posttranslationally modified by SUMO. Mol. Cell. Biol. 29, 714–725 (2009).
Yu, W. et al. Poly(ADP-ribosyl)ation regulates CTCF-dependent chromatin insulation. Nature Genet. 36, 1105–1110 (2004).
Witcher, M. & Emerson, B. M. Epigenetic silencing of the p16(INK4a) tumor suppressor is associated with loss of CTCF binding and a chromatin boundary. Mol. Cell 34, 271–284 (2009).
Ong, C. T., Van Bortle, K., Ramos, E. & Corces, V. G. Poly(ADP-ribosyl)ation regulates insulator function and intrachromosomal interactions in Drosophila. Cell 155, 148–159 (2013). This work shows that poly(ADP-ribosyl)ation regulates CTCF-mediated intrachromosomal interactions and its association with other architectural protein in D. melanogaster.
Zlatanova, J. & Caiafa, P. CTCF and its protein partners: divide and rule? J. Cell Sci. 122, 1275–1284 (2009).
Xiao, T., Wallace, J. & Felsenfeld, G. Specific sites in the C terminus of CTCF interact with the SA2 subunit of the cohesin complex and are required for cohesin-dependent insulation activity. Mol. Cell. Biol. 31, 2174–2183 (2011).
Wendt, K. S. et al. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature 451, 796–801 (2008).
Parelho, V. et al. Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell 132, 422–433 (2008).
Rubio, E. D. et al. CTCF physically links cohesin to chromatin. Proc. Natl. Acad. Sci. USA 105, 8309–8314 (2008).
Stedman, W. et al. Cohesins localize with CTCF at the KSHV latency control region and at cellular c-myc and H19/Igf2 insulators. EMBO J. 27, 654–666 (2008).
Galli, G. G. et al. Genomic and proteomic analyses of Prdm5 reveal interactions with insulator binding proteins in embryonic stem cells. Mol. Cell. Biol. 33, 4504–4516 (2013).
Xie, D. et al. Dynamic trans-acting factor colocalization in human cells. Cell 155, 713–724 (2013).
Hadjur, S. et al. Cohesins form chromosomal cis-interactions at the developmentally regulated IFNG locus. Nature 460, 410–413 (2009).
Hou, C., Dale, R. & Dean, A. Cell type specificity of chromatin organization mediated by CTCF and cohesin. Proc. Natl Acad. Sci. USA 107, 3651–3656 (2010).
Nativio, R. et al. Cohesin is required for higher-order chromatin conformation at the imprinted IGF2–H19 locus. PLoS Genet. 5, e1000739 (2009).
Kirkland, J. G., Raab, J. R. & Kamakaka, R. T. TFIIIC bound DNA elements in nuclear organization and insulation. Biochim. Biophys. Acta 1829, 418–424 (2013).
Noma, K., Cam, H. P., Maraia, R. J. & Grewal, S. I. A role for TFIIIC transcription factor complex in genome organization. Cell 125, 859–872 (2006).
Lunyak, V. V. et al. Developmentally regulated activation of a SINE B2 repeat as a domain boundary in organogenesis. Science 317, 248–251 (2007).
Raab, J. R. et al. Human tRNA genes function as chromatin insulators. EMBO J. 31, 330–350 (2012).
Carriere, L. et al. Genomic binding of Pol III transcription machinery and relationship with TFIIS transcription factor distribution in mouse embryonic stem cells. Nucleic Acids Res. 40, 270–283 (2012).
Oler, A. J. et al. Human RNA polymerase III transcriptomes and relationships to Pol II promoter chromatin and enhancer-binding factors. Nature Struct. Mol. Biol. 17, 620–628 (2010).
Moqtaderi, Z. et al. Genomic binding profiles of functionally distinct RNA polymerase III transcription complexes in human cells. Nature Struct. Mol. Biol. 17, 635–640 (2010). In this study, genome-wide mapping of distinct components of the Pol III complex in human cells reveals the association of TFIIIC with many ETC loci that are located near CTCF-binding sites.
Lei, E. P. & Corces, V. G. RNA interference machinery influences the nuclear organization of a chromatin insulator. Nature Genet. 38, 936–941 (2006).
Yao, H. et al. Mediation of CTCF transcriptional insulation by DEAD-box RNA-binding protein p68 and steroid receptor RNA activator SRA. Genes Dev. 24, 2543–2555 (2010).
Sun, S. et al. Jpx RNA activates Xist by evicting CTCF. Cell 153, 1537–1551 (2013). This work shows that the interaction of the Jpx ncRNA with CTCF is necessary for the eviction of CTCF from the promoter to facilitate Xist transcription.
Zhao, Z. et al. Circular chromosome conformation capture (4C) uncovers extensive networks of epigenetically regulated intra- and interchromosomal interactions. Nature Genet. 38, 1341–1347 (2006).
Splinter, E. et al. CTCF mediates long-range chromatin looping and local histone modification in the β-globin locus. Genes Dev. 20, 2349–2354 (2006).
Blanton, J., Gaszner, M. & Schedl, P. Protein:protein interactions and the pairing of boundary elements in vivo. Genes Dev. 17, 664–675 (2003).
Byrd, K. & Corces, V. G. Visualization of chromatin domains created by the gypsy insulator of Drosophila. J. Cell Biol. 162, 565–574 (2003).
Kellum, R. & Schedl, P. A position-effect assay for boundaries of higher order chromosomal domains. Cell 64, 941–950 (1991).
Oki, M. & Kamakaka, R. T. Barrier function at HMR. Mol. Cell 19, 707–716 (2005).
Huang, S., Li, X., Yusufzai, T. M., Qiu, Y. & Felsenfeld, G. USF1 recruits histone modification complexes and is critical for maintenance of a chromatin barrier. Mol. Cell. Biol. 27, 7991–8002 (2007).
Guelen, L. et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 453, 948–951 (2008).
Handoko, L. et al. CTCF-mediated functional chromatin interactome in pluripotent cells. Nature Genet. 43, 630–638 (2011). This paper shows that CTCF-mediated chromatin interactions may separate chromatin domains and facilitate enhancer–promoter contacts.
Essafi, A. et al. A Wt1-controlled chromatin switching mechanism underpins tissue-specific Wnt4 activation and repression. Dev. Cell 21, 559–574 (2011).
Taslim, C. et al. Integrated analysis identifies a class of androgen-responsive genes regulated by short combinatorial long-range mechanism facilitated by CTCF. Nucleic Acids Res. 40, 4754–4764 (2012).
Kim, Y. J., Cecchini, K. R. & Kim, T. H. Conserved, developmentally regulated mechanism couples chromosomal looping and heterochromatin barrier activity at the homeobox gene A locus. Proc. Natl Acad. Sci. USA 108, 7391–7396 (2011).
Schwartz, Y. B. et al. Nature and function of insulator protein binding sites in the Drosophila genome. Genome Res. 22, 2188–2198 (2012).
Van Bortle, K. et al. Drosophila CTCF tandemly aligns with other insulator proteins at the borders of H3K27me3 domains. Genome Res. 22, 2176–2187 (2012).
Li, H. B. et al. Insulators, not Polycomb response elements, are required for long-range interactions between Polycomb targets in Drosophila melanogaster. Mol. Cell. Biol. 31, 616–625 (2011).
Wood, A. M. et al. Regulation of chromatin organization and inducible gene expression by a Drosophila insulator. Mol. Cell 44, 29–38 (2011).
Xie, X. et al. Systematic discovery of regulatory motifs in conserved regions of the human genome, including thousands of CTCF insulator sites. Proc. Natl Acad. Sci. USA 104, 7145–7150 (2007).
Yang, J., Ramos, E. & Corces, V. G. The BEAF-32 insulator coordinates genome organization and function during the evolution of Drosophila species. Genome Res. 22, 2199–2207 (2012).
Sanyal, A., Lajoie, B. R., Jain, G. & Dekker, J. The long-range interaction landscape of gene promoters. Nature 489, 109–113 (2012). By mapping long-range interactions between distal elements and transcription start sites, this study finds that CTCF-binding sites are enriched at many enhancer elements and that enhancer–promoter interactions may not be disrupted by intervening CTCF-binding sites.
Shen, Y. et al. A map of the cis-regulatory sequences in the mouse genome. Nature 488, 116–120 (2012).
Majumder, P. & Boss, J. M. CTCF controls expression and chromatin architecture of the human major histocompatibility complex class II locus. Mol. Cell. Biol. 30, 4211–4223 (2010).
Majumder, P., Gomez, J. A., Chadwick, B. P. & Boss, J. M. The insulator factor CTCF controls MHC class II gene expression and is required for the formation of long-distance chromatin interactions. J. Exp. Med. 205, 785–798 (2008).
Xu, Z., Wei, G., Chepelev, I., Zhao, K. & Felsenfeld, G. Mapping of INS promoter interactions reveals its role in long-range regulation of SYT8 transcription. Nature Struct. Mol. Biol. 18, 372–378 (2011).
Golan-Mashiach, M. et al. Identification of CTCF as a master regulator of the clustered protocadherin genes. Nucleic Acids Res. 40, 3378–3391 (2012).
Kehayova, P., Monahan, K., Chen, W. & Maniatis, T. Regulatory elements required for the activation and repression of the protocadherin-α gene cluster. Proc. Natl Acad. Sci. USA 108, 17195–17200 (2011).
Guo, Y. et al. CTCF/cohesin-mediated DNA looping is required for protocadherin α promoter choice. Proc. Natl Acad. Sci. USA 109, 21081–21086 (2012).
Monahan, K. et al. Role of CCCTC binding factor (CTCF) and cohesin in the generation of single-cell diversity of protocadherin-α gene expression. Proc. Natl Acad. Sci. USA 109, 9125–9130 (2012).
Hirayama, T., Tarusawa, E., Yoshimura, Y., Galjart, N. & Yagi, T. CTCF is required for neural development and stochastic expression of clustered Pcdh genes in neurons. Cell Rep. 2, 345–357 (2012).
Liu, Z., Scannell, D. R., Eisen, M. B. & Tjian, R. Control of embryonic stem cell lineage commitment by core promoter factor, TAF3. Cell 146, 720–731 (2011). This study shows that CTCF directly recruits TAF3 to promoter distal sites that act as enhancers for endoderm lineage differentiation in ESCs.
Bossen, C., Mansson, R. & Murre, C. Chromatin topology and the regulation of antigen receptor assembly. Annu. Rev. Immunol. 30, 337–356 (2012).
Guo, C. et al. Two forms of loops generate the chromatin conformation of the immunoglobulin heavy-chain gene locus. Cell 147, 332–343 (2011).
Degner, S. C. et al. CCCTC-binding factor (CTCF) and cohesin influence the genomic architecture of the Igh locus and antisense transcription in pro-B cells. Proc. Natl Acad. Sci. USA 108, 9566–9571 (2011).
Shih, H. Y. et al. Tcra gene recombination is supported by a Tcra enhancer- and CTCF-dependent chromatin hub. Proc. Natl Acad. Sci. USA 109, E3493–E3502 (2012).
Guo, C. et al. CTCF-binding elements mediate control of V(D)J recombination. Nature 477, 424–430 (2011). This study shows that binding of CTCF to a key V(D)J recombination regulatory region mediates chromatin looping to regulate ordered and lineage-specific rearrangement of the Igh locus.
Ribeiro de Almeida, C. et al. The DNA-binding protein CTCF limits proximal Vκ recombination and restricts κ enhancer interactions to the immunoglobulin κ light chain locus. Immunity 35, 501–513 (2011).
Stadhouders, R. et al. Dynamic long-range chromatin interactions control Myb proto-oncogene transcription during erythroid development. EMBO J. 31, 986–999 (2012).
Paredes, S. H., Melgar, M. F. & Sethupathy, P. Promoter-proximal CCCTC-factor binding is associated with an increase in the transcriptional pausing index. Bioinformatics 29, 1485–1487 (2013).
Shukla, S. et al. CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature 479, 74–79 (2011). This paper shows that DNA methylation and binding of CTCF at exons regulate alternative splicing by controlling the elongation rate of Pol II.
Hou, C., Li, L., Qin, Z. S. & Corces, V. G. Gene density, transcription, and insulators contribute to the partition of the Drosophila genome into physical domains. Mol. Cell 48, 471–484 (2012).
Sexton, T. et al. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell 148, 458–472 (2012).
Dixon, J. R. et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376–380 (2012). This study shows that the mammalian genome is organized into TADs that are separated by boundaries enriched for CTCF and other architectural proteins.
Nora, E. P. et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 485, 381–385 (2012).
Zuin, J. et al. Cohesin and CTCF differentially affect chromatin architecture and gene expression in human cells. Proc. Natl. Acad. Sci. USA 111, 996–1001 (2013).
Sofueva, S. et al. Cohesin-mediated interactions organize chromosomal domain architecture. EMBO J. 32, 3119–3129 (2013).
Seitan, V. C. et al. Cohesin-based chromatin interactions enable regulated gene expression within preexisting architectural compartments. Genome Res. 23, 2066–2077 (2013). References 96–99 show that CTCF and cohesin are required to maintain the organization of TADs.
Lin, Y. C. et al. Global changes in the nuclear positioning of genes and intra- and interdomain genomic interactions that orchestrate B cell fate. Nature Immunol. 13, 1196–1204 (2012).
Phillips-Cremins, J. E. et al. Architectural protein subclasses shape 3D organization of genomes during lineage commitment. Cell 153, 1281–1295 (2013).
Naumova, N. et al. Organization of the mitotic chromosome. Science 342, 948–953 (2013).
Acknowledgements
Work in the authors' laboratory is supported by US Public Health Service Award R01 GM035463 from the US National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Chromosome conformation capture
-
(3C). A ligation-based technique that is used to map interactions between two specific genomic regions.
- ChIP-exo
-
An extension of chromatin immunoprecipitation followed by sequencing (ChIP–seq) that includes exonuclease trimming after ChIP to increase the resolution of the mapped transcription factor bound sites.
- Circular chromosome conformation capture
-
(4C). The combination of inverse PCR and high-throughput sequencing with the chromosome conformation capture (3C) technique that allows the profiling of chromatin interactions between a known specific locus and multiple unknown sites.
- DNase I hypersensitive sites
-
Chromosomal regions that are readily degraded by deoxyribonuclease I (DNase I) owing to decreased nucleosome occupancy. These sites are associated with open chromatin conformation and the binding of transcription factors.
- Nuclear lamina
-
A scaffold of proteins comprised mainly of lamin A/C and B that is predominantly found in the nuclear periphery; it is associated with the inner surface of the nuclear membrane.
- Chromatin interaction analysis with paired-end tag sequencing
-
(ChIA–PET). A technique used to determine the chromosomal interactions that are mediated by a specific chromatin-binding protein by combining chromatin immunoprecipitation with a chromosome conformation capture (3C)-type analysis.
- Chromosome conformation capture carbon copy
-
(5C). A technique used to profile all chromatin interactions in specific regions of the genome by the hybridization of a mixture of DNA primers to chromosome conformation capture (3C) templates followed by high-throughput sequencing.
- Mediator complex
-
The ~30-subunit co-activator complex that is required for successful transcription of RNA polymerase II (Pol II) promoters of metazoans genes. Its interaction with Pol II and site-specific factors facilitates enhancer–promoter communication.
- Adaptive response
-
The acquired immune response to the specific antigen presented on a pathogen that typically triggers immunological memory.
- Pro-B cells
-
B cells at their earliest developmental stage in the bone marrow that are defined as the CD19+ cytoplasmic IgM− or B220+CD43+ population and that have incomplete rearrangement of the immunoglobulin heavy chain.
- Double-positive thymocytes
-
Immature T cells characterized by the expression of CD4 and CD8 cell surface markers that will differentiate into single-positive thymocytes after their T cell receptors interact with self-peptide major histocompatibility complex ligands in the thymus.
- Pre-pro-B cells
-
The lymphoid progenitors found in the bone marrow that contain the CLP-2s surface marker and lack heavy-chain diversity (D)–joining (J) rearrangements.
- Hi-C
-
An extension of the chromosome conformation capture (3C) technique that incorporates a biotin-labelled nucleotide at the ligation junction to allow selective purification of chimeric DNA ligated products for high-throughput sequencing. This method generates matrices of interaction frequencies across the genome.
Rights and permissions
About this article
Cite this article
Ong, CT., Corces, V. CTCF: an architectural protein bridging genome topology and function. Nat Rev Genet 15, 234–246 (2014). https://doi.org/10.1038/nrg3663
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg3663