Key Points
-
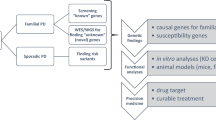
Motor neuron diseases (MNDs) are an etiologically heterogeneous group of disorders that are characterized by muscle weakness and/or spastic paralysis, which results from the selective degeneration of lower motor neurons and/or upper motor neurons, respectively.
-
The MNDs currently being investigated are: amyotrophic lateral sclerosis (ALS), hereditary spastic paraplegia (HSP), primary lateral sclerosis (PLS), spinal muscular atrophy (SMA), spinal bulbar muscular atrophy (SBMA) and lethal congenital contracture syndrome (LCCS).
-
ALS is the most common adult-onset MND for which there is no therapeutic treatment currently available. The hallmark of this disease is the selective death of motor neurons in the brain and spinal cord, which leads to the paralysis of voluntary muscles.
-
Family-based linkage studies have led to the identification of eight genes for ALS. The protein products of these mutated genes are superoxide dismutase 1 (SOD1), alsin, senataxin, vesicle-associated membrane protein-associated protein B (VAPB), angiogenin, dynactin, TAR DNA-binding protein 43 (TDP43) and FUS.
-
Mutations in the SOD1 gene are the most common genetic cause of familial ALS and account for 15–20% of autosomal dominant familial ALS cases (1–2% of all ALS cases).
-
HSPs are the second most important group of MNDs in terms of the number of mutations identified and the resulting insights into the pathogenesis of MND. The 45 spastic paraplegia loci and 20 causative genes reported so far suggest various pathogenic mechanisms, including axonal transport, membrane trafficking and mitochondrial dysfunction.
-
RNA-processing defects are observed in several MNDs, and in ALS the TARDBP and FUS genes have provided direct links to defects in RNA processing as a broad pathway that contributes to motor neuron degeneration (however, this is not the case for HSP).
Abstract
The past few years have seen the identification of dozens of genes with causal roles in motor neuron diseases (MNDs), particularly for amyotrophic lateral sclerosis and hereditary spastic paraplegia. Although many additional MND genes remain to be identified, the accumulated genetic evidence has already provided new insights into MND pathogenesis, which adds to the well-established involvement of superoxide dismutase 1 (SOD1) mutations. The pathways that have been recently implicated include those that affect RNA processing, axonal transport and mitochondrial function. The functional classes of MND genes identified so far are likely to aid the selection of high-priority candidate genes for future investigation, including those for so-called sporadic cases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rosen, D. R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993). This report describes the original discovery of SOD1 mutations in FALS cases. SOD1 was the first ALS causative gene to be identified.
Wroe, R., Wai-Ling Butler, A., Andersen, P. M., Powell, J. F. & Al-Chalabi, A. ALSOD: the Amyotrophic Lateral Sclerosis Online Database. Amyotroph. Lateral Scler. 9, 249–250 (2008).
Reaume, A. G. et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nature Genet. 13, 43–47 (1996).
Gurney, M. E. et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994). This paper reports the first generation and description of an ALS transgenic mouse model.
Rothstein, J. D. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann. Neurol. 65, S3–S9 (2009).
Li, X. et al. Mutant copper-zinc superoxide dismutase associated with amyotrophic lateral sclerosis binds to adenine/uridine-rich stability elements in the vascular endothelial growth factor 3′-untranslated region. J. Neurochem. 108, 1032–1044 (2009).
Lambrechts, D. et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nature Genet. 34, 383–394 (2003).
Pramatarova, A., Laganiere, J., Roussel, J., Brisebois, K. & Rouleau, G. A. Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment. J. Neurosci. 21, 3369–3374 (2001). This paper offers evidence that SOD1 toxicity is not cell autonomous.
Gong, Y. H., Parsadanian, A. S., Andreeva, A., Snider, W. D. & Elliott, J. L. Restricted expression of G86R Cu/Zn superoxide dismutase in astrocytes results in astrocytosis but does not cause motoneuron degeneration. J. Neurosci. 20, 660–665 (2000). This paper offers evidence surrounding the crucial contribution of astrocytes during the pathogenesis of ALS.
Yamanaka, K. et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nature Neurosci. 11, 251–253 (2008).
Lobsiger, C. S. & Cleveland, D. W. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nature Neurosci. 10, 1355–1360 (2007).
Andersen, P. M. Amyotrophic lateral sclerosis associated with mutations in the CuZn superoxide dismutase gene. Curr. Neurol. Neurosci. Rep. 6, 37–46 (2006).
Andersen, P. M. et al. Autosomal recessive adult-onset amyotrophic lateral sclerosis associated with homozygosity for Asp90Ala CuZn-superoxide dismutase mutation. A clinical and genealogical study of 36 patients. Brain 119, 1153–1172 (1996).
Andersen, P. M. et al. Amyotrophic lateral sclerosis associated with homozygosity for an Asp90Ala mutation in CuZn-superoxide dismutase. Nature Genet. 10, 61–66 (1995).
Hand, C. K. et al. Compound heterozygous D90A and D96N SOD1 mutations in a recessive amyotrophic lateral sclerosis family. Ann. Neurol. 49, 267–271 (2001).
Zinman, L. et al. A mechanism for low penetrance in an ALS family with a novel SOD1 deletion. Neurology 72, 1153–1159 (2009).
Mount, S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 10, 459–472 (1982).
Ezzi, S. A., Urushitani, M. & Julien, J. P. Wild-type superoxide dismutase acquires binding and toxic properties of ALS-linked mutant forms through oxidation. J. Neurochem. 102, 170–178 (2007).
Kabashi, E., Valdmanis, P. N., Dion, P. & Rouleau, G. A. Oxidized/misfolded superoxide dismutase-1: the cause of all amyotrophic lateral sclerosis? Ann. Neurol. 62, 553–559 (2007).
Rakhit, R. et al. An immunological epitope selective for pathological monomer-misfolded SOD1 in ALS. Nature Med. 13, 754–759 (2007).
Jonsson, P. A. et al. Minute quantities of misfolded mutant superoxide dismutase-1 cause amyotrophic lateral sclerosis. Brain 127, 73–88 (2004).
Urushitani, M., Ezzi, S. A. & Julien, J. P. Therapeutic effects of immunization with mutant superoxide dismutase in mice models of amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA 104, 2495–2500 (2007).
Arai, T. et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 351, 602–611 (2006).
Neumann, M. et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006). The two reports above showed that protein aggregates in ALS contain TDP43 and therefore prompted ongoing and extensive investigations into the role of this protein in ALS.
Mackenzie, I. R. et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann. Neurol. 61, 427–434 (2007).
Kabashi, E. et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nature Genet. 40, 572–574 (2008). This paper describes eight missense mutations in TARDBP in patients with SALS and FALS and shows that the mutations correspond with the accumulation of a lower-molecular-weight TDP43 fragment.
Sreedharan, J. et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319, 1668–1672 (2008). The authors identify three missense mutations in TARDBP in patients with ALS (including one large family) and provide proof of the effects of these mutations by injecting chick embryos.
Daoud, H. et al. Contribution of TARDBP mutations to sporadic amyotrophic lateral sclerosis. J. Med. Genet. 46, 112–114 (2009).
Lagier-Tourenne, C. & Cleveland, D. W. Rethinking ALS: the FUS about TDP-43. Cell 136, 1001–1004 (2009).
Corrado, L. et al. High frequency of TARDBP gene mutations in Italian patients with amyotrophic lateral sclerosis. Hum. Mutat. 30, 688–694 (2009).
Benajiba, L. et al. TARDBP mutations in motoneuron disease with frontotemporal lobar degeneration. Ann. Neurol. 65, 470–473 (2009).
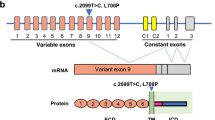
Buratti, E. et al. TDP-43 binds heterogeneous nuclear ribonucleoprotein A/B through its C-terminal tail: an important region for the inhibition of cystic fibrosis transmembrane conductance regulator exon 9 splicing. J. Biol. Chem. 280, 37572–37584 (2005).
Valdmanis, P. N., Daoud, H., Dion, P. A. & Rouleau, G. A. Recent advances in the genetics of amyotrophic lateral sclerosis. Curr. Neurol. Neurosci. Rep. 9, 198–205 (2009).
Kwiatkowski, T. J. Jr et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323, 1205–1208 (2009).
Vance, C. et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323, 1208–1211 (2009). The two papers above describe FUS mutations in the ALS6 locus in cases of FALS.
Abalkhail, H., Mitchell, J., Habgood, J., Orrell, R. & de Belleroche, J. A new familial amyotrophic lateral sclerosis locus on chromosome 16q12.1–16q12.2. Am. J. Hum. Genet. 73, 383–389 (2003).
Ruddy, D. M. et al. Two families with familial amyotrophic lateral sclerosis are linked to a novel locus on chromosome 16q. Am. J. Hum. Genet. 73, 390–396 (2003).
Sapp, P. C. et al. Identification of two novel loci for dominantly inherited familial amyotrophic lateral sclerosis. Am. J. Hum. Genet. 73, 397–403 (2003).
Belzil, V. V. et al. Mutations in FUS cause FALS and SALS in French and French Canadian populations. Neurology 9 Sep 2009 (doi:10.1212/WNL.0b013e3181bbfeef).
Buratti, E. & Baralle, F. E. Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front. Biosci. 13, 867–878 (2008).
Hadano, S. et al. A gene encoding a putative GTPase regulator is mutated in familial amyotrophic lateral sclerosis 2. Nature Genet. 29, 166–173 (2001).
Hentati, A. et al. Linkage of recessive familial amyotrophic lateral sclerosis to chromosome 2q33–q35. Nature Genet. 7, 425–428 (1994).
Gros-Louis, F. et al. Als2 mRNA splicing variants detected in KO mice rescue severe motor dysfunction phenotype in Als2 knock-down zebrafish. Hum. Mol. Genet. 17, 2691–2702 (2008).
Eymard-Pierre, E. et al. Novel missense mutation in ALS2 gene results in infantile ascending hereditary spastic paralysis. Ann. Neurol. 59, 976–980 (2006).
Gros-Louis, F. et al. An ALS2 gene mutation causes hereditary spastic paraplegia in a Pakistani kindred. Ann. Neurol. 53, 144–145 (2003).
Otomo, A. et al. ALS2, a novel guanine nucleotide exchange factor for the small GTPase Rab5, is implicated in endosomal dynamics. Hum. Mol. Genet. 12, 1671–1687 (2003).
Chen, Y. Z. et al. DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4). Am. J. Hum. Genet. 74, 1128–1135 (2004).
Hentati, A. et al. Linkage of a commoner form of recessive amyotrophic lateral sclerosis to chromosome 15q15–q22 markers. Neurogenetics 2, 55–60 (1998).
Nishimura, A. L. et al. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am. J. Hum. Genet. 75, 822–831 (2004).
Greenway, M. J. et al. ANG mutations segregate with familial and 'sporadic' amyotrophic lateral sclerosis. Nature Genet. 38, 411–413 (2006).
Fernandez-Santiago, R. et al. Identification of novel angiogenin (ANG) gene missense variants in German patients with amyotrophic lateral sclerosis. J. Neurol. 256, 1432–1459 (2009).
Gellera, C. et al. Identification of new ANG gene mutations in a large cohort of Italian patients with amyotrophic lateral sclerosis. Neurogenetics 9, 33–40 (2008).
Paubel, A. et al. Mutations of the ANG gene in French patients with sporadic amyotrophic lateral sclerosis. Arch. Neurol. 65, 1333–1336 (2008).
Wu, D. et al. Angiogenin loss-of-function mutations in amyotrophic lateral sclerosis. Ann. Neurol. 62, 609–617 (2007).
van Es, M. A. et al. A case of ALS-FTD in a large FALS pedigree with a K17I ANG mutation. Neurology 72, 287–288 (2009).
Sebastia, J. et al. Angiogenin protects motoneurons against hypoxic injury. Cell Death Differ. (2009).
Puls, I. et al. Mutant dynactin in motor neuron disease. Nature Genet. 33, 455–456 (2003).
Munch, C. et al. Heterozygous R1101K mutation of the DCTN1 gene in a family with ALS and FTD. Ann. Neurol. 58, 777–780 (2005).
Hand, C. K. et al. A novel locus for familial amyotrophic lateral sclerosis, on chromosome 18q. Am. J. Hum. Genet. 70, 251–256 (2002).
Siddique, T. et al. X-linked dominant locus for late-onset familial amyotrophic lateral sclerosis. Am. J. Hum. Genet. 63 (Suppl.), A308 (1998).
Neary, D., Snowden, J. & Mann, D. Frontotemporal dementia. Lancet Neurol. 4, 771–780 (2005).
Talbot, K. & Ansorge, O. Recent advances in the genetics of amyotrophic lateral sclerosis and frontotemporal dementia: common pathways in neurodegenerative disease. Hum. Mol. Genet. 15, R182–R187 (2006).
Hosler, B. A. et al. Linkage of familial amyotrophic lateral sclerosis with frontotemporal dementia to chromosome 9q21–q22. JAMA 284, 1664–1669 (2000).
Morita, M. et al. A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology 66, 839–844 (2006).
Valdmanis, P. N. et al. Three families with amyotrophic lateral sclerosis and frontotemporal dementia with evidence of linkage to chromosome 9p. Arch. Neurol. 64, 240–245 (2007).
Vance, C. et al. Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2–213. Brain 129, 868–876 (2006).
Le Ber, I. et al. Chromosome 9p-linked families with frontotemporal dementia associated with motor neuron disease. Neurology 72, 1669–1676 (2009).
Luty, A. A. et al. Pedigree with frontotemporal lobar degeneration — motor neuron disease and Tar DNA binding protein-43 positive neuropathology: genetic linkage to chromosome 9. BMC Neurol. 8, 32 (2008). The six references above describe families with ALS and FTD that were mapped to the chromosome 9p locus.
Hutton, M. et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705 (1998).
Zarranz, J. J. et al. A novel mutation (K317M) in the MAPT gene causes FTDP and motor neuron disease. Neurology 64, 1578–1585 (2005).
Cruts, M. et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442, 920–924 (2006).
Watts, G. D. et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nature Genet. 36, 377–381 (2004).
Parkinson, N. et al. ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B). Neurology 67, 1074–1077 (2006).
Spina, S. et al. Clinicopathologic features of frontotemporal dementia with Progranulin sequence variation. Neurology 68, 820–827 (2007).
Valdmanis, P. N. & Rouleau, G. A. Genetics of familial amyotrophic lateral sclerosis. Neurology 70, 144–152 (2008).
Wills, A. M. et al. A large-scale international meta-analysis of paraoxonase gene polymorphisms in sporadic ALS. Neurology 73, 16–24 (2009).
McCarthy, M. I. et al. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nature Rev. Genet. 9, 356–369 (2008).
Schymick, J. C. et al. Genome-wide genotyping in amyotrophic lateral sclerosis and neurologically normal controls: first stage analysis and public release of data. Lancet Neurol. 6, 322–328 (2007).
Dunckley, T. et al. Whole-genome analysis of sporadic amyotrophic lateral sclerosis. N. Engl. J. Med. 357, 775–788 (2007).
van Es, M. A. et al. ITPR2 as a susceptibility gene in sporadic amyotrophic lateral sclerosis: a genome-wide association study. Lancet Neurol. 6, 869–877 (2007).
van Es, M. A. et al. Genetic variation in DPP6 is associated with susceptibility to amyotrophic lateral sclerosis. Nature Genet. 40, 29–31 (2008).
Cronin, S. et al. A genome-wide association study of sporadic ALS in a homogenous Irish population. Hum. Mol. Genet. 17, 768–774 (2008).
Del Bo, R. et al. DPP6 gene variability confers increased risk of developing sporadic amyotrophic lateral sclerosis in Italian patients. J. Neurol. Neurosurg. Psychiatry 79, 1085 (2008).
Cronin, S., Tomik, B., Bradley, D. G., Slowik, A. & Hardiman, O. Screening for replication of genome-wide SNP associations in sporadic ALS. Eur. J. Hum. Genet. 17, 213–218 (2009).
Chiò, A. et al. A two-stage genome-wide association study of sporadic amyotrophic lateral sclerosis. Hum. Mol. Genet. 18, 1524–1532 (2009).
Simpson, C. L. et al. Variants of the elongator protein 3 (ELP3) gene are associated with motor neuron degeneration. Hum. Mol. Genet. 18, 472–481 (2009).
van Es, M. A. et al. Genome-wide association study identifies 19p13.3 (UNC13A) and 9p21.2 as susceptibility loci for sporadic amyotrophic lateral sclerosis. Nature Genet. 6 Sep 2009 (doi:10.1038/ng.442).
Kaneko, K., Saito, F., Sunohara, N. & Ikeuchi, T. Cytogenetic analysis of 23 Japanese patients with amyotrophic lateral sclerosis. Clin. Genet. 47, 158–160 (1995).
Meyer, T. et al. High rate of constitutional chromosomal rearrangements in apparently sporadic ALS. Neurology 60, 1348–1350 (2003).
Blauw, H. M. et al. Copy-number variation in sporadic amyotrophic lateral sclerosis: a genome-wide screen. Lancet Neurol. 7, 319–326 (2008). This paper presents the first examination of potential CNVs in ALS.
Cronin, S. et al. Analysis of genome-wide copy number variation in Irish and Dutch ALS populations. Hum. Mol. Genet. 17, 3392–3398 (2008).
Hazan, J. et al. Spastin, a new AAA protein, is altered in the most frequent form of autosomal dominant spastic paraplegia. Nature Genet. 23, 296–303 (1999). This study shows that mutations in SPAST are the most common cause of autosomal dominant HSP.
Hollenbeck, P. J. & Saxton, W. M. The axonal transport of mitochondria. J. Cell Sci. 118, 5411–5419 (2005).
van Niekerk, E. A. et al. Sumoylation in axons triggers retrograde transport of the RNA-binding protein La. Proc. Natl Acad. Sci. USA 104, 12913–12918 (2007).
Svenson, I. K., Ashley-Koch, A. E., Pericak-Vance, M. A. & Marchuk, D. A. A second leaky splice-site mutation in the spastin gene. Am. J. Hum. Genet. 69, 1407–1409 (2001).
Solowska, J. M. et al. Quantitative and functional analyses of spastin in the nervous system: implications for hereditary spastic paraplegia. J. Neurosci. 28, 2147–2157 (2008).
Reid, E. et al. The hereditary spastic paraplegia protein spastin interacts with the ESCRT-III complex-associated endosomal protein CHMP1B. Hum. Mol. Genet. 14, 19–38 (2005).
Yang, D. et al. Structural basis for midbody targeting of spastin by the ESCRT-III protein CHMP1B. Nature Struct. Mol. Biol. 15, 1278–1286 (2008).
Zhao, X. et al. Mutations in a newly identified GTPase gene cause autosomal dominant hereditary spastic paraplegia. Nature Genet. 29, 326–331 (2001).
Namekawa, M. et al. SPG3A is the most frequent cause of hereditary spastic paraplegia with onset before age 10 years. Neurology 66, 112–114 (2006). This paper reports that mutations in ATL1 account for ∼10% of cases of autosomal dominant HSP.
Namekawa, M. et al. Mutations in the SPG3A gene encoding the GTPase atlastin interfere with vesicle trafficking in the ER/Golgi interface and Golgi morphogenesis. Mol. Cell Neurosci. 35, 1–13 (2007).
Zhu, P. P., Soderblom, C., Tao-Cheng, J. H., Stadler, J. & Blackstone, C. SPG3A protein atlastin-1 is enriched in growth cones and promotes axon elongation during neuronal development. Hum. Mol. Genet. 15, 1343–1353 (2006).
Evans, K. et al. Interaction of two hereditary spastic paraplegia gene products, spastin and atlastin, suggests a common pathway for axonal maintenance. Proc. Natl Acad. Sci. USA 103, 10666–10671 (2006).
Reid, E. et al. A kinesin heavy chain (KIF5A) mutation in hereditary spastic paraplegia (SPG10). Am. J. Hum. Genet. 71, 1189–1194 (2002).
Goizet, C. et al. Complicated forms of autosomal dominant hereditary spastic paraplegia are frequent in SPG10. Hum. Mutat. 30, E376–E385 (2009).
Rainier, S., Chai, J. H., Tokarz, D., Nicholls, R. D. & Fink, J. K. NIPA1 gene mutations cause autosomal dominant hereditary spastic paraplegia (SPG6). Am. J. Hum. Genet. 73, 967–971 (2003).
Reed, J. A. et al. A novel NIPA1 mutation associated with a pure form of autosomal dominant hereditary spastic paraplegia. Neurogenetics 6, 79–84 (2005).
Goytain, A., Hines, R. M., El-Husseini, A. & Quamme, G. A. NIPA1(SPG6), the basis for autosomal dominant form of hereditary spastic paraplegia, encodes a functional Mg2+ transporter. J. Biol. Chem. 282, 8060–8068 (2007).
Zhao, J. et al. Hereditary spastic paraplegia-associated mutations in the NIPA1 gene and its Caenorhabditis elegans homolog trigger neural degeneration in vitro and in vivo through a gain-of-function mechanism. J. Neurosci. 28, 13938–13951 (2008).
Wang, X., Shaw, W. R., Tsang, H. T., Reid, E. & O'Kane, C. J. Drosophila spichthyin inhibits BMP signaling and regulates synaptic growth and axonal microtubules. Nature Neurosci. 10, 177–185 (2007).
Hanein, S. et al. Identification of the SPG15 gene, encoding spastizin, as a frequent cause of complicated autosomal-recessive spastic paraplegia, including Kjellin syndrome. Am. J. Hum. Genet. 82, 992–1002 (2008).
Patel, H. et al. SPG20 is mutated in Troyer syndrome, an hereditary spastic paraplegia. Nature Genet. 31, 347–348 (2002).
Simpson, M. A. et al. Maspardin is mutated in mast syndrome, a complicated form of hereditary spastic paraplegia associated with dementia. Am. J. Hum. Genet. 73, 1147–1156 (2003).
Bakowska, J. C., Jupille, H., Fatheddin, P., Puertollano, R. & Blackstone, C. Troyer syndrome protein spartin is mono-ubiquitinated and functions in EGF receptor trafficking. Mol. Biol. Cell 18, 1683–1692 (2007).
Hanna, M. C. & Blackstone, C. Interaction of the SPG21 protein ACP33/maspardin with the aldehyde dehydrogenase ALDH16A1. Neurogenetics 10, 217–228 (2009).
Hehr, U. et al. Long-term course and mutational spectrum of spatacsin-linked spastic paraplegia. Ann. Neurol. 62, 656–665 (2007).
Stevanin, G. et al. Mutations in SPG11, encoding spatacsin, are a major cause of spastic paraplegia with thin corpus callosum. Nature Genet. 39, 366–372 (2007).
Casari, G. et al. Spastic paraplegia and OXPHOS impairment caused by mutations in paraplegin, a nuclear-encoded mitochondrial metalloprotease. Cell 93, 973–983 (1998).
Wilkinson, P. A. et al. A clinical, genetic and biochemical study of SPG7 mutations in hereditary spastic paraplegia. Brain 127, 973–980 (2004).
Koppen, M., Metodiev, M. D., Casari, G., Rugarli, E. I. & Langer, T. Variable and tissue-specific subunit composition of mitochondrial m-AAA protease complexes linked to hereditary spastic paraplegia. Mol. Cell Biol. 27, 758–767 (2007).
Ferreirinha, F. et al. Axonal degeneration in paraplegin-deficient mice is associated with abnormal mitochondria and impairment of axonal transport. J. Clin. Invest. 113, 231–242 (2004).
Hansen, J. J. et al. Hereditary spastic paraplegia SPG13 is associated with a mutation in the gene encoding the mitochondrial chaperonin Hsp60. Am. J. Hum. Genet. 70, 1328–1332 (2002).
Hewamadduma, C. A. et al. HSP60 is a rare cause of hereditary spastic paraparesis, but may act as a genetic modifier. Neurology 70, 1717–1718 (2008).
Jouet, M. et al. X-linked spastic paraplegia (SPG1), MASA syndrome and X-linked hydrocephalus result from mutations in the L1 gene. Nature Genet. 7, 402–407 (1994).
Saugier-Veber, P. et al. X-linked spastic paraplegia and Pelizaeus–Merzbacher disease are allelic disorders at the proteolipid protein locus. Nature Genet. 6, 257–262 (1994).
Hortsch, M. Structural and functional evolution of the L1 family: are four adhesion molecules better than one? Mol. Cell Neurosci. 15, 1–10 (2000).
Inoue, K. PLP1-related inherited dysmyelinating disorders: Pelizaeus–Merzbacher disease and spastic paraplegia type 2. Neurogenetics 6, 1–16 (2005).
Tsaousidou, M. K. et al. Sequence alterations within CYP7B1 implicate defective cholesterol homeostasis in motor-neuron degeneration. Am. J. Hum. Genet. 82, 510–515 (2008).
Lin, P. et al. A missense mutation in SLC33A1, which encodes the acetyl-CoA transporter, causes autosomal-dominant spastic paraplegia (SPG42). Am. J. Hum. Genet. 83, 752–759 (2008).
Hirabayashi, Y., Kanamori, A., Nomura, K. H. & Nomura, K. The acetyl-CoA transporter family SLC33. Pflugers Arch. 447, 760–762 (2004).
Kanamori, A. et al. Expression cloning and characterization of a cDNA encoding a novel membrane protein required for the formation of O-acetylated ganglioside: a putative acetyl-CoA transporter. Proc. Natl Acad. Sci. USA 94, 2897–2902 (1997).
Orthmann-Murphy, J. L. et al. Hereditary spastic paraplegia is a novel phenotype for GJA12/GJC2 mutations. Brain 132, 426–438 (2009).
Valdmanis, P. N. et al. Mutations in the KIAA0196 gene at the SPG8 locus cause hereditary spastic paraplegia. Am. J. Hum. Genet. 80, 152–161 (2007).
Windpassinger, C. et al. Heterozygous missense mutations in BSCL2 are associated with distal hereditary motor neuropathy and Silver syndrome. Nature Genet. 36, 271–276 (2004).
Bohan, T. P. & Azizi, P. Allan–Herndon–Dudley syndrome: should the locus for this hereditary spastic paraplegia be designated SPG 22? Arch. Neurol. 61, 1470–1471 (2004).
Beetz, C. et al. REEP1 mutation spectrum and genotype/phenotype correlation in hereditary spastic paraplegia type 31. Brain 131, 1078–1086 (2008).
Zuchner, S. et al. A new locus for dominant hereditary spastic paraplegia maps to chromosome 2p12. Neurogenetics 7, 127–129 (2006).
Rainier, S. et al. Neuropathy target esterase gene mutations cause motor neuron disease. Am. J. Hum. Genet. 82, 780–785 (2008).
Gordon, P. H., Cheng, B., Katz, I. B., Mitsumoto, H. & Rowland, L. P. Clinical features that distinguish PLS, upper motor neuron-dominant ALS, and typical ALS. Neurology 72, 1948–1952 (2009).
Valdmanis, P. N., Dupre, N. & Rouleau, G. A. A locus for primary lateral sclerosis on chromosome 4ptel–4p16.1. Arch. Neurol. 65, 383–386 (2008). The authors describe a French-Canadian family with PLSA1 that maps to chromosome 4p.
Mintchev, N., Zamba-Papanicolaou, E., Kleopa, K. A. & Christodoulou, K. A novel ALS2 splice-site mutation in a Cypriot juvenile-onset primary lateral sclerosis family. Neurology 72, 28–32 (2009).
Panzeri, C. et al. The first ALS2 missense mutation associated with JPLS reveals new aspects of alsin biological function. Brain 129, 1710–1719 (2006).
Yang, Y. et al. The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nature Genet. 29, 160–165 (2001).
Lorson, C. L. et al. SMN oligomerization defect correlates with spinal muscular atrophy severity. Nature Genet. 19, 63–66 (1998).
Monani, U. R. et al. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 8, 1177–1183 (1999).
Lorson, C. L. & Androphy, E. J. An exonic enhancer is required for inclusion of an essential exon in the SMA-determining gene SMN. Hum. Mol. Genet. 9, 259–265 (2000).
Pellizzoni, L., Kataoka, N., Charroux, B. & Dreyfuss, G. A novel function for SMN, the spinal muscular atrophy disease gene product, in pre-mRNA splicing. Cell 95, 615–624 (1998).
Lefebvre, S. et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80, 155–165 (1995). This paper shows that loss-of-function mutations or deletions of the SMN1 gene cause SMA.
Corcia, P. et al. The importance of the SMN genes in the genetics of sporadic ALS. Amyotroph. Lateral Scler. 6 Mar 2009 (doi:10.1080/17482960902759162).
La Spada, A. R., Wilson, E. M., Lubahn, D. B., Harding, A. E. & Fischbeck, K. H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352, 77–79 (1991). The authors identified a trinucleotide CAG repeat expansion in the first exon of the AR gene as being responsible for SBMA.
Katsuno, M. et al. Leuprorelin rescues polyglutamine-dependent phenotypes in a transgenic mouse model of spinal and bulbar muscular atrophy. Nature Med. 9, 768–773 (2003).
Makela-Bengs, P. et al. Assignment of the disease locus for lethal congenital contracture syndrome to a restricted region of chromosome 9q34, by genome scan using five affected individuals. Am. J. Hum. Genet. 63, 506–516 (1998).
Nousiainen, H. O. et al. Mutations in mRNA export mediator GLE1 result in a fetal motoneuron disease. Nature Genet. 40, 155–157 (2008).
Narkis, G. et al. Lethal contractural syndrome type 3 (LCCS3) is caused by a mutation in PIP5K1C, which encodes PIPKI gamma of the phophatidylinsitol pathway. Am. J. Hum. Genet. 81, 530–539 (2007).
Narkis, G. et al. Lethal congenital contractural syndrome type 2 (LCCS2) is caused by a mutation in ERBB3 (Her3), a modulator of the phosphatidylinositol-3-kinase/Akt pathway. Am. J. Hum. Genet. 81, 589–595 (2007).
Chow, C. Y. et al. Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am. J. Hum. Genet. 84, 85–88 (2009).
Yeo, G., Holste, D., Kreiman, G. & Burge, C. B. Variation in alternative splicing across human tissues. Genome Biol. 5, R74 (2004).
Salinas, S., Proukakis, C., Crosby, A. & Warner, T. T. Hereditary spastic paraplegia: clinical features and pathogenetic mechanisms. Lancet Neurol. 7, 1127–1138 (2008).
Boillee, S., Vande Velde, C. & Cleveland, D. W. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52, 39–59 (2006).
Lin, M. T. & Beal, M. F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795 (2006).
Hirano, A., Donnenfeld, H., Sasaki, S. & Nakano, I. Fine structural observations of neurofilamentous changes in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 43, 461–470 (1984).
Hirano, A. et al. Fine structural study of neurofibrillary changes in a family with amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 43, 471–480 (1984).
Khabazian, I. et al. Isolation of various forms of sterol β-D-glucoside from the seed of Cycas circinalis: neurotoxicity and implications for ALS–parkinsonism dementia complex. J. Neurochem. 82, 516–528 (2002).
Horner, R. D. et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology 61, 742–749 (2003).
Li, C. Y. & Sung, F. C. Association between occupational exposure to power frequency electromagnetic fields and amyotrophic lateral sclerosis: a review. Am. J. Ind. Med. 43, 212–220 (2003).
Qureshi, M. M. et al. Analysis of factors that modify susceptibility and rate of progression in amyotrophic lateral sclerosis (ALS). Amyotroph. Lateral Scler. 7, 173–182 (2006).
Doi, H. et al. Motor neuron disorder simulating ALS induced by chronic inhalation of pyrethroid insecticides. Neurology 67, 1894–1895 (2006).
Weisskopf, M. G. et al. Prospective study of chemical exposures and amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 80, 558–561 (2009).
Chio, A., Benzi, G., Dossena, M., Mutani, R. & Mora, G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain 128, 472–476 (2005).
Chio, A. et al. ALS in Italian professional soccer players: the risk is still present and could be soccer-specific. Amyotroph. Lateral Scler. 10, 205–209 (2009).
Valenti, M. et al. Amyotrophic lateral sclerosis and sports: a case–control study. Eur. J. Neurol. 12, 223–225 (2005).
Chen, H., Richard, M., Sandler, D. P., Umbach, D. M. & Kamel, F. Head injury and amyotrophic lateral sclerosis. Am. J. Epidemiol. 166, 810–816 (2007).
Weisskopf, M. G. et al. Prospective study of cigarette smoking and amyotrophic lateral sclerosis. Am. J. Epidemiol. 160, 26–33 (2004).
Santos-Reboucas, C. B. & Pimentel, M. M. Implication of abnormal epigenetic patterns for human diseases. Eur. J. Hum. Genet. 15, 10–17 (2007).
Migliore, L. & Coppedè, F. Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat. Res. 667, 82–97 (2008).
Oates, N. & Pamphlett, R. An epigenetic analysis of SOD1 and VEGF in ALS. Amyotroph. Lateral Scler. 8, 83–86 (2007).
Morahan, J. M., Yu, B., Trent, R. J. & Pamphlett, R. Are metallothionein genes silenced in ALS? Toxicol. Lett. 168, 83–87 (2007).
Acknowledgements
The authors wish to thank C. Vande Velde and I. A. Meijer for their careful and insightful reading of the manuscript; their ideas and suggestions were welcomed and appreciated. G.A.R. has received MND-related funding from the Canadian Institutes of Health, the Muscular Dystrophy Association ALS Division, the ALS Association and the ALS Society of Canada.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary information S1 (table)
(PDF 296 kb)
Related links
Glossary
- Linkage study
-
A method of searching for the chromosomal location of a gene by looking for co-segregation of the disease with genetic markers of known chromosomal location within families.
- Epigenetics
-
Changes in gene expression that are stable through cell division but do not involve changes in the underlying DNA sequence. The best-studied example is cellular differentiation, but environmental factors, such as maternal nutrition, can influence epigenetic programming.
- Reactive oxygen species
-
Ions or small molecules that include oxygen ions, free radicals and peroxides, both inorganic and organic.
- Hu-antigen R
-
An RNA-stabilizing protein that is a member of the embryonic lethal abnormal visual (ELAV) family. These proteins recognize the 3′ UTR sequences of mRNAs, in particular the adenine/uridine-rich elements, the widespread occurrence of which suggests that they are involved in the regulation of many biological processes.
- Astrocyte
-
One of the three main cell types in the brain, the others being neurons and oligodendrocytes. Astrocytes act as a scaffold that maintains brain structure and they can alter the extracellular milieu and ionic concentration through the expression of various transporters and channel proteins. They support the functions of neurons and oligodendrocytes.
- Cre recombinase
-
A type I topoisomerase from the P1 bacteriophage that catalyses the site-specific recombination of DNA between loxP sites. It binds to the loxP sites to allow DNA that is cloned between the sites to be removed.
- Microglia
-
Small neuroglial cells of the central nervous system. They have long processes and ameboid and phagocytic activity at sites of neural damage or inflammation.
- Schwann cell
-
A type of non-neuronal brain cell that lacks axons and dendrites and forms axons in the peripheral nervous system.
- Microglia activation
-
Microglia can be activated by several factors, including glutamate receptor agonists, pro-inflammatory cytokines, cell necrosis factors and lipopolysaccharide. Once activated, the cells undergo key morphological changes, including the secretion of cytotoxic factors, recruitment molecules and pro-inflammatory molecules. In addition, activated microglia undergo proliferation to increase their numbers.
- Penetrance
-
The proportion of individuals with a specific genotype who manifest the genotype at the phenotypic level. If the penetrance of a disease allele is 100%, all individuals who carry that allele will express the associated disorder and the genotype is said to be 'completely penetrant'.
- Polymorphism
-
The contemporary definition is any site in the DNA sequence that is present in the population in more than one state. By contrast, the traditional definition is an allele with a population frequency of between >1% and <99%.
- Compound heterozygote
-
A situation in which an individual is heterozygous for two different mutations at the same locus.
- Proband
-
In a family study, the individual who is first identified in the family as having the disease under study.
- Heterogeneous nuclear ribonucleoprotein
-
A complex of RNA and protein that is present in the nucleus during transcription and post-transcriptional modification of pre-mRNA. Such complexes serve as a signal that the pre-mRNA is not yet fully processed and ready for export to the cytoplasm.
- Homozygosity mapping
-
An approach for detecting rare disease-promoting variants. This method detects extensive homozygous haplotypes that are hundreds of kilobases or more in length and that are unique to, or enriched in, affected individuals.
- Retrograde motor
-
Motor proteins bind and transport several different cargoes in nerve cells, including organelles, polymers and vesicles containing neurotransmitters. Retrograde transport runs towards the minus end of the axons.
- Frontal lobe
-
An area of the brain located at the front of each cerebral hemisphere that is involved in higher mental functions. The executive functions of the frontal lobe include the ability to recognize future consequences, override and suppress unacceptable social responses, and determine similarities and differences between things or events.
- Anterior temporal lobe
-
The temporal lobes are regions of the cerebral cortex that are located beneath the Sylvian fissure on both the left and right hemispheres of the brain. The anterior part of the lobes is involved in visual processing and object perception and recognition.
- Haploinsufficiency
-
A condition in a diploid organism in which a single functional copy of a gene results in a phenotype, such as a disease.
- Association studies
-
A gene-discovery strategy that compares allele frequencies in cases and controls to assess the contribution of genetic variants to phenotypes in specific populations.
- Meta-analysis
-
An approach that combines the results of several studies that address a set of related research hypotheses to overcome the problem of reduced statistical power in studies with small sample sizes.
- Population stratification
-
A population that contains several subpopulations that differ in their genetic characteristics.
- Genome-wide association studies
-
The examination of DNA variation (typically SNPs) across the whole genome in a large number of individuals who have been matched for population ancestry and assessed for a disease or trait of interest. Correlations between variants and the trait are used to locate genetic risk factors.
- Microsatellite
-
A class of repetitive DNA that is made up of repeats that are 2–8 nucleotides in length. Microsatellites can be highly polymorphic and are frequently used as molecular markers in population genetics studies.
- 1000 Genomes Project
-
An international research effort, launched in 2008, to establish by far the most detailed catalogue of human genetic variation. Plans are to sequence the genomes of at least 1,000 anonymous participants of different ethnic groups over the next 3 years using newly developed technologies.
- Copy number variant
-
A DNA sequence variant (including deletions and duplications) in which the result is a departure from the expected diploid representation of the DNA sequence.
- Balanced translocation
-
A translocation between non-homologous chromosomes in which the exchange occurs with no gain or loss of genetic material.
- Midbody
-
A transient organelle-like structure that is formed during mammalian cell division and persists until just before the complete separation of the dividing cells.
- Myelin
-
An electrically insulating material that usually forms a layer around the axon of a neuron. It is essential for the proper functioning of the nervous system. Schwann cells supply the myelin for peripheral neurons, whereas oligodendrocytes supply it to neurons of the central nervous system.
- Anterior horn
-
The ventral column of grey matter in the spinal cord that contains the cell bodies of motor (efferent) neurons.
- Cristae
-
Internal compartments that are formed by the inner membranes of mitochondria. They contain several key proteins for aerobic respiration, including ATP synthase and various cytochromes.
Rights and permissions
About this article
Cite this article
Dion, P., Daoud, H. & Rouleau, G. Genetics of motor neuron disorders: new insights into pathogenic mechanisms. Nat Rev Genet 10, 769–782 (2009). https://doi.org/10.1038/nrg2680
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg2680