Key Points
-
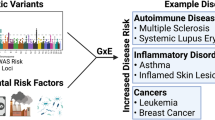
Human epidemiological studies and animal investigations provide compelling evidence that prenatal and early postnatal environmental factors influence the adult risk of developing various chronic diseases, such as cancer, cardiovascular disease, diabetes, obesity and behavioural disorders such as schizophrenia.
-
The developmental origins of adult-onset disease hypothesis proposes that the evolution of developmental plasticity, which enables an organism to adapt to environmental signals during early life, can also increase the risk of developing chronic diseases when there is a mismatch between the perceived environment and that which is encountered in adulthood.
-
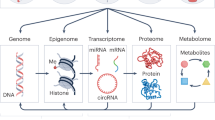
Epigenetics is the study of alterations in gene expression that occur not by changing the DNA sequence, but by modifying DNA methylation and remodelling chromatin structure.
-
Prenatal and postnatal environmental exposures could be linked to phenotypic changes later in life through the alteration of the epigenetic marks that regulate the functional output of the information that is stored in the genome.
-
In support of this postulate, maternal methyl-donor supplementation during pregnancy with folic acid, vitamin B12, choline and betaine was shown to effect the phenotype of the Avy (viable yellow agouti) offspring by directly altering the epigenome.
-
Studies with the fungicide vinclozolin demonstrate that heritable environmentally induced epigenetic modifications can also underlie transgenerational alterations in phenotype.
-
Novel genome-wide experimental and bioinformatic techniques are now being used to identify epigenetically labile genes in humans. Such approaches will hopefully allow for the development of unique epigenetic-based diagnostic, prevention and therapeutic strategies for human diseases.
Abstract
Epidemiological evidence increasingly suggests that environmental exposures early in development have a role in susceptibility to disease in later life. In addition, some of these environmental effects seem to be passed on through subsequent generations. Epigenetic modifications provide a plausible link between the environment and alterations in gene expression that might lead to disease phenotypes. An increasing body of evidence from animal studies supports the role of environmental epigenetics in disease susceptibility. Furthermore, recent studies have demonstrated for the first time that heritable environmentally induced epigenetic modifications underlie reversible transgenerational alterations in phenotype. Methods are now becoming available to investigate the relevance of these phenomena to human disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Yajnik, C. S. Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries. J. Nutr. 134, 205–210 (2004).
Barker, D. J. The developmental origins of chronic adult disease. Acta Paediatr. Suppl. 93, 26–33 (2004).
Painter, R. C., Roseboom, T. J. & Bleker, O. P. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod. Toxicol. 20, 345–352 (2005).
Gluckman, P. D., Hanson, M. A. & Beedle, A. S. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am. J. Hum. Biol. 19, 1–19 (2007). References 1–4 discuss evidence for the early origins of the adult disease susceptibility hypothesis.
St Clair, D. et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. JAMA 294, 557–562 (2005).
van Os, J. & Selten, J. P. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br. J. Psychiatry 172, 324–326 (1998). References 5 and 6 discuss epidemiological evidence that the adult incidence of schizophrenia is significantly increased in humans who were exposed prenatally to famine conditions.
Li, E. Chromatin modification and epigenetic reprogramming in mammalian development. Nature Rev. Genet. 3, 662–673 (2002).
Klose, R. J. & Bird, A. P. Genomic DNA methylation: the mark and its mediators. Trends Biochem. Sci. 31, 89–97 (2006).
Talbert, P. B. & Henikoff, S. Spreading of silent chromatin: inaction at a distance. Nature Rev. Genet. 7, 793–803 (2006).
Richards, E. J. Inherited epigenetic variation — revisiting soft inheritance. Nature Rev. Genet. 7, 395–401 (2006).
Thorvaldsen, J. L., Verona, R. I. & Bartolomei, M. S. X-tra! X-tra! News from the mouse X chromosome. Dev. Biol. 298, 344–353 (2006).
Huynh, K. D. & Lee, J. T. X-chromosome inactivation: a hypothesis linking ontogeny and phylogeny. Nature Rev. Genet. 6, 410–418 (2005).
Lewis, A. & Reik, W. How imprinting centres work. Cytogenet. Genome Res. 113, 81–89 (2006).
Reik, W. & Walter, J. Genomic imprinting: parental influence on the genome. Nature Rev. Genet. 2, 21–32 (2001).
Falls, J. G., Pulford, D. J., Wylie, A. A. & Jirtle, R. L. Genomic imprinting: implications for human disease. Am. J. Pathol. 154, 635–647 (1999).
Murphy, S. K. & Jirtle, R. L. Imprinting evolution and the price of silence. BioEssays 25, 577–588 (2003).
Slotkin, R. K. & Martienssen, R. Transposable elements and the epigenetic regulation of the genome. Nature Rev. Genet. 8, 272–285 (2007).
Wolff, G. L., Kodell, R. L., Moore, S. R. & Cooney, C. A. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 12, 949–957 (1998).
Waterland, R. A. & Jirtle, R. L. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol. Cell. Biol. 23, 5293–5300 (2003). This study demonstrates that maternal methyl donor supplementation during gestation can alter offspring phenotype by methylating the epigenome.
Dolinoy, D. C., Weidman, J. R., Waterland, R. A. & Jirtle, R. L. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ. Health Perspect. 114, 567–572 (2006).
Waterland, R. A. et al. Maternal methyl supplements increase offspring DNA methylation at Axin fused. Genesis 44, 401–406 (2006).
Waterland, R. A., Lin, J. R., Smith, C. A. & Jirtle, R. L. Post-weaning diet affects genomic imprinting at the insulin-like growth factor 2 (IGF2) locus. Hum. Mol. Genet. 15, 705–716 (2006).
Li, S. et al. Neonatal diethylstilbestrol exposure induces persistent elevation of c-fos expression and hypomethylation in its exon-4 in mouse uterus. Mol. Carcinog. 38, 78–84 (2003).
Ho, S. M., Tang, W. Y., Belmonte de Frausto, J. & Prins, G. S. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 66, 5624–5632 (2006).
Anway, M. D. & Skinner, M. K. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology 147, S43–S49 (2006).
Weaver, I. C. G. et al. Epigenetic programming by maternal behavior. Nature Neurosci. 7, 847–854 (2004).
Weaver, I. C. et al. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J. Neurosci. 25, 11045–11054 (2005).
Niemitz, E. L. & Feinberg, A. P. Epigenetics and assisted reproductive technology: a call for investigation. Am. J. Hum. Genet. 74, 599–609 (2004).
Rossignol, S. et al. The epigenetic imprinting defect of patients with Beckwith–Wiedemann syndrome born after assisted reproductive technology is not restricted to the 11p15 region. J. Med. Genet. 43, 902–907 (2006).
Koturbash, I. et al. Epigenetic dysregulation underlies radiation-induced transgenerational genome instability in vivo. Int. J. Radiat. Oncol. Biol. Phys. 66, 327–330 (2006).
Morgan, H. D., Sutherland, H. G. E., Martin, D. I. K. & Whitelaw, E. Epigenetic inheritance at the agouti locus in the mouse. Nature Genet. 23, 314–318 (1999). This study demonstrates the maternal inheritance of an epigenetic modification at the agouti locus in mice.
Lane, N. et al. Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis 35, 88–93 (2003).
Rakyan, V. K. et al. Transgenerational inheritance of epigenetic states at the murine Axin(Fu) allele occurs after maternal and paternal transmission. Proc. Natl Acad. Sci. USA 100, 2538–2543 (2003).
Anway, M. D., Cupp, A. S., Uzumcu, M. & Skinner, M. K. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308, 1466–1469 (2005). This study demonstrates the ability of environmental factors to induce an epigenetic transgenerational disease phenotype for four generations.
Pembrey, M. E. et al. Sex-specific, male-line transgenerational responses in humans. Eur. J. Hum. Genet. 14, 159–166 (2006). This study demonstrates an inherited disease phenotype in humans that is potentially induced by an epigenetic phenomena.
Vasicek, T. J. et al. Two dominant mutations in the mouse fused gene are the result of transposon insertions. Genetics 147, 777–786 (1997).
Suter, C. M., Martin, D. I. & Ward, R. L. Germline epimutation of MLH1 in individuals with multiple cancers. Nature Genet. 36, 497–501 (2004).
Chan, T. L. et al. Heritable germline epimutation of MSH2 in a family with hereditary nonpolyposis colorectal cancer. Nature Genet. 38, 1178–1183 (2006).
Esteller, M. Epigenetics provides a new generation of oncogenes and tumour-suppressor genes. Br. J. Cancer 94, 179–183 (2006).
Yoo, C. B. & Jones, P. A. Epigenetic therapy of cancer: past, present and future. Nature Rev. Drug Discov. 5, 37–50 (2006).
Baylin, S. B. & Ohm, J. E. Epigenetic gene silencing in cancer — a mechanism for early oncogenic pathway addiction? Nature Rev. Cancer 6, 107–116 (2006).
Duhl, D. M., Vrieling, H., Miller, K. A., Wolff, G. L. & Barsh, G. S. Neomorphic agouti mutations in obese yellow mice. Nature Genet. 8, 59–65 (1994). These authors show that the Avy allele results from the insertion of an intracisternal A particle upstream of the agouti gene.
Druker, R., Bruxner, T. J., Lehrbach, N. J. & Whitelaw, E. Complex patterns of transcription at the insertion site of a retrotransposon in the mouse. Nucl. Acids Res. 32, 5800–5808 (2004).
Rakyan, V. K., Blewitt, M. E., Druker, R., Preis, J. I. & Whitelaw, E. Metastable epialleles in mammals. Trends Genet. 18, 348–351 (2002).
Druker, R. & Whitelaw, E. Retrotransposon-derived elements in the mammalian genome: a potential source of disease. Inherit. Metab. Dis. 27, 319–330 (2004).
Miltenberger, R. J., Mynatt, R. L., Wilkinson, J. E. & Woychik, R. P. The role of the agouti gene in the Yellow Obese Syndrome. J. Nutr. 127, 1902S–1907S (1997).
Cooney, C. A., Dave, A. A. & Wolff, G. L. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J. Nutr. 132, 2393S–2400S (2002).
Cropley, J. E., Suter, C. M., Beckman, K. B. & Martin, D. I. Germ-line epigenetic modification of the murine Avy allele by nutritional supplementation. Proc. Natl Acad. Sci. USA 103, 17308–17312 (2006).
Haig, D. & Graham, C. Genomic imprinting and the strange case of the insulin-like growth factor II receptor. Cell 64, 1045–1046 (1991). These authors propose that genomic imprinting evolved because of a parental genetic battle to control the amount of nutrients that is extracted from the mother by the offspring.
Wilkins, J. F. & Haig, D. What good is genomic imprinting: the function of parent-specific gene expression. Nature Rev. Genet. 4, 359–368 (2003).
DeChiara, T. M., Robertson, E. & Efstratiadis, A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell 64, 849–859 (1991).
Barlow, D. P., Stoger, R., Herrmann, B. G., Saito, K. & Schweifer, N. The mouse insulin-like growth factor type-2 receptor is imprinted and closely linked to the Tme locus. Nature 349, 84–87 (1991). References 51 and 52 report the first-identified imprinted genes.
Killian, J. K. et al. M6p/IGF2R imprinting evolution in mammals. Mol. Cell 5, 707–716 (2000). This paper demonstrates that genomic imprinting evolved approximately 180 million years ago with the advent of live birth in therian mammals.
Evans, H. K., Weidman, J. R., Cowley, D. O. & Jirtle, R. L. Comparative phylogenetic analysis of Blcap/Nnat reveals eutherian-specific imprinted gene. Mol. Biol. Evol. 22, 1740–1748 (2005).
Weidman, J. R., Maloney, K. A. & Jirtle, R. L. Comparative phylogenetic analysis reveals multiple non-imprinted isoforms of opossum DLK1. Mamm. Genome 17, 157–167 (2006).
Suzuki, S. et al. Genomic imprinting of IGF2, p57(KIP2) and PEG1/MEST in a marsupial, the tammar wallaby. Mech. Dev. 122, 213–222 (2005).
Killian, J. K. et al. Divergent evolution in M6P/IGF2R imprinting from the Jurassic to the Quaternary. Hum. Mol. Genet. 10, 1721–1728 (2001).
De Souza, A. T., Hankins, G. R., Washington, M. K., Orton, T. C. & Jirtle, R. L. M6P/IGF2R gene is mutated in human hepatocellular carcinomas with loss of heterozygosity. Nature Genet. 11, 447–449 (1995).
Weksberg, R., Shuman, C. & Smith, A. C. Beckwith–Wiedemann syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 137, 12–23 (2005).
Kantor, B., Shemer, R. & Razin, A. The Prader-Willi–Angelman imprinted domain and its control center. Cytogenet. Genome Res. 113, 300–305 (2006).
Badcock, C. & Crespi, B. Imbalanced genomic imprinting in brain development: an evolutionary basis for the aetiology of autism. J. Evol. Biol. 19, 1007–1032 (2006). These authors propose that human neurological disorders, such as autism, result from an imbalanced expression of imprinted genes during development.
Morison, I. M., Ramsay, J. P. & Spencer, H. G. A census of mammalian imprinting. Trends Genet. 21, 457–465 (2005).
Feinberg, A. P., Ohlsson, R. & Henikoff, S. The epigenetic progenitor origin of human cancer. Nature Rev. Genet. 7, 21–33 (2006).
Feinberg, A. P. A genetic approach to cancer epigenetics. Cold Spring Harb. Symp. Quant. Biol. 70, 335–341 (2005).
Knudson, A. G. Two genetic hits (more or less) to cancer. Nature Rev. Cancer 1, 157–162 (2001).
Cui, H. et al. Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science 299, 1753–1755 (2003). This paper reports that some humans have IGF2 LOI in peripheral lymphocytes, which is correlated with biallelic expression in normal colonic mucosa and a personal history of colorectal cancer.
Cruz-Correa, M. et al. Loss of imprinting of insulin growth factor II gene: a potential heritable biomarker for colon neoplasia predisposition. Gastroenterology 126, 964–970 (2004).
Jirtle, R. L. IGF2 loss of imprinting: a potential heritable risk factor for colorectal cancer. Gastroenterology 126, 1190–1193 (2004).
Oates, N. A. et al. Increased DNA methylation at the AXIN1 gene in a monozygotic twin from a pair discordant for a caudal duplication anomaly. Am. J. Hum. Genet. 79, 155–162 (2006).
Ikeda, M., Tamura, M., Yamashita, J., Suzuki, C. & Tomita, T. Repeated in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure affects male gonads in offspring, leading to sex ratio changes in F2 progeny. Toxicol. Appl. Pharmacol. 206, 351–355 (2005).
Blatt, J., Van Le, L., Weiner, T. & Sailer, S. Ovarian carcinoma in an adolescent with transgenerational exposure to diethylstilbestrol. J. Pediatr. Hematol. Oncol. 25, 635–636 (2003).
Dubrova, Y. E. Radiation-induced transgenerational instability. Oncogene 22, 7087–7093 (2003).
Cheng, R. Y., Hockman, T., Crawford, E., Anderson, L. M. & Shiao, Y. H. Epigenetic and gene expression changes related to transgenerational carcinogenesis. Mol. Carcinog. 40, 1–11 (2004).
Hemmings, D. G., Veerareddy, S., Baker, P. N. & Davidge, S. T. Increased myogenic responses in uterine but not mesenteric arteries from pregnant offspring of diet-restricted rat dams. Biol. Reprod. 72, 997–1003 (2005).
Ferguson, L. R., Karunasinghe, N. & Philpott, M. Epigenetic events and protection from colon cancer in New Zealand. Environ. Mol. Mutagen. 44, 36–43 (2004).
Csaba, G. & Karabelyos, C. Transgenerational effect of a single neonatal benzpyrene treatment (imprinting) on the sexual behavior of adult female rats. Hum. Exp. Toxicol. 16, 553–556 (1997).
Fujii, T. Transgenerational effects of maternal exposure to chemicals on the functional development of the brain in the offspring. Cancer Causes Control 8, 524–528 (1997).
Brucker-Davis, F. Effects of environmental synthetic chemicals on thyroid function. Thyroid 8, 827–856 (1998).
Giusti, R. M., Iwamoto, K. & Hatch, E. E. Diethylstilbestrol revisited: a review of the long-term health effects. Ann. Intern. Med. 122, 778–788 (1995).
Klip, H. et al. Hypospadias in sons of women exposed to diethylstilbestrol in utero: a cohort study. Lancet 359, 1102–1107 (2002).
Parks, L. G. et al. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol. Sci. 58, 339–349 (2000).
Steinhardt, G. F. Endocrine disruption and hypospadias. Adv. Exp. Med. Biol. 545, 203–215 (2004).
Ruden, D. M., Xiao, L., Garfinkel, M. D. & Lu, X. Hsp90 and environmental impacts on epigenetic states: a model for the trans-generational effects of diethylstibesterol on uterine development and cancer. Hum. Mol. Genet. 14, R149–R155 (2005).
Matta, M. B., Linse, J., Cairncross, C., Francendese, L. & Kocan, R. M. Reproductive and transgenerational effects of methylmercury or Aroclor 1268 on Fundulus heteroclitus. Environ. Toxicol. Chem. 20, 327–335 (2001).
Omholt, S. W. & Amdam, G. V. Epigenetic regulation of aging in honeybee workers. Sci. Aging Knowledge Environ. 2004, pe28 (2004).
Ottinger, M. A. et al. Assessing the consequences of the pesticide methoxychlor: neuroendocrine and behavioral measures as indicators of biological impact of an estrogenic environmental chemical. Brain Res. Bull. 65, 199–209 (2005).
Seidl, M. D., Paul, R. J. & Pirow, R. Effects of hypoxia acclimation on morpho-physiological traits over three generations of Daphnia magna. J. Exp. Biol. 208, 2165–2175 (2005).
Foran, C. M., Peterson, B. N. & Benson, W. H. Transgenerational and developmental exposure of Japanese medaka (Oryzias latipes) to ethinylestradiol results in endocrine and reproductive differences in the response to ethinylestradiol as adults. Toxicol. Sci. 68, 389–402 (2002).
Anderson, C. M., Lopez, F., Zimmer, A. & Benoit, J. N. Placental insufficiency leads to developmental hypertension and mesenteric artery dysfunction in two generations of Sprague–Dawley rat offspring. Biol. Reprod. 74, 538–544 (2006).
Csaba, G. & Inczefi-Gonda, A. Transgenerational effect of a single neonatal benzpyrene treatment on the glucocorticoid receptor of the rat thymus. Hum. Exp. Toxicol. 17, 88–92 (1998).
Newbold, R. R., Padilla-Banks, E. & Jefferson, W. N. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology 147, S11–S17 (2006).
Popova, N. V. Transgenerational effect of orthoaminoasotoluol in mice. Cancer Lett. 46, 203–206 (1989).
Zambrano, E. et al. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J. Physiol. 566, 225–236 (2005).
Cesani, M. F. et al. Effect of undernutrition on the cranial growth of the rat. An intergenerational study. Cells Tissues Organs 174, 129–135 (2003).
Turusov, V. S., Nikonova, T. V. & Parfenov, Y. Increased multiplicity of lung adenomas in five generations of mice treated with benz(a)pyrene when pregnant. Cancer Lett. 55, 227–231 (1990).
Anway, M. D., Leathers, C. & Skinner, M. K. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology 147, 5515–5523 (2006).
Chang, H. S., Anway, M. D., Rekow, S. S. & Skinner, M. K. Transgenerational epigenetic imprinting of the male germline by endocrine disruptor exposure during gonadal sex determination. Endocrinology 147, 5524–5541 (2006). This report demonstrates the ability of vinclozolin to induce the reprogramming of the germ line, and the formation of genes and DNA sequences that contain paternal-allele alterations in DNA methylation associated with transgenerational disease.
Durcova-Hills, G. et al. Influence of sex chromosome constitution on the genomic imprinting of germ cells. Proc. Natl Acad. Sci. USA 103, 11184–11188 (2006).
Forum, T. C. News and Information. J. Radiol. Prot. 25, 499–502 (2005).
Allegrucci, C., Thurston, A., Lucas, E. & Young, L. Epigenetics and the germline. Reproduction 129, 137–149 (2005).
McCarrey, J. R., Geyer, C. B. & Yoshioka, H. Epigenetic regulation of testis-specific gene expression. Ann. NY Acad. Sci. 1061, 226–242 (2005).
Trasler, J. M. Origin and roles of genomic methylation patterns in male germ cells. Semin. Cell Dev. Biol. 9, 467–474 (1998).
Weaver, I. C., Meaney, M. J. & Szyf, M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc. Natl Acad. Sci. USA 103, 3480–3485 (2006).
Hurst, G. D. & Werren, J. H. The role of selfish genetic elements in eukaryotic evolution. Nature Rev. Genet. 2, 597–606 (2001).
Bestor, T. H. Cytosine methylation mediates sexual conflict. Trends Genet. 19, 185–190 (2003).
Luedi, P. P., Hartemink, A. J. & Jirtle, R. L. Genome-wide prediction of imprinted murine genes. Genome Res. 15, 875–884 (2005). These authors demonstrate that imprinted genes and their parental expression bias can be predicted genome-wide with the use of machine learning algorithms.
Waddington, C. H. Organisers and Genes (Cambridge Univ. Press, Cambridge, 1940).
Holliday, R. & Pugh, J. E. DNA modification mechanisms and gene activity during development. Science 187, 226–232 (1975).
Willard, H. F., Brown, C. J., Carrel, L., Hendrich, B. & Miller, A. P. Epigenetic and chromosomal control of gene expression: molecular and genetic analysis of X chromosome inactivation. Cold Spring Harb. Symp. Quant. Biol. 58, 315–322 (1993).
Monk, M. Genomic imprinting. Genes Dev. 2, 921–925 (1988).
Wolffe, A. P. & Matzke, M. A. Epigenetics: regulation through repression. Science 286, 481–486 (1999).
Murrell, A., Rakyan, V. K. & Beck, S. From genome to epigenome. Hum. Mol. Genet. 14, R3–R10 (2005).
Kishino, T. Imprinting in neurons. Cytogenet. Genome Res. 113, 209–214 (2006).
Vu, T. H., Jirtle, R. L. & Hoffman, A. R. Cross-species clues of an epigenetic imprinting regulatory code for the IGF2R gene. Cytogenet. Genome Res. 113, 202–208 (2006).
Mayer, W., Niveleau, A., Walter, J., Fundele, R. & Haaf, T. Demethylation of the zygotic paternal genome. Nature 403, 501–552 (2000).
Acknowledgements
The authors wish to thank D. Dolinoy for critically reading the manuscript and for her helpful suggestions. We thank J. Griffin for assistance in the preparation of this manuscript. This work was supported by grants from the US Department of Energy and the US National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Epigenetic
-
Refers to mitotically or meiotically heritable changes in gene expression that do not involve a change in DNA sequence.
- DNA methylation
-
DNA methylation occurs predominantly in repetitive genomic regions that contain CpG residues. DNA methylation represses transcription directly by inhibiting the binding of specific transcription factors, and indirectly by recruiting methyl-CpG-binding proteins and their associated repressive chromatin-remodelling activities.
- Histone modifications
-
Histones undergo post-translational modifications that alter their interaction with DNA and nuclear proteins. In particular, the tails of histones H3 and H4 can be covalently modified at several residues. Modifications of the tail include methylation, acetylation, phosphorylation and ubiquitination, and influence several biological processes, including gene expression, DNA repair and chromosome condensation.
- MicroRNAs
-
Endogenous small RNAs of ∼22 nucleotides in length that act as a cellular rheostat for fine-tuning gene expression during development and differentiation. They target the 3′ UTRs of mRNAs with which they share partial sequence complementarity, leading to post-transcriptional gene silencing through translational repression. When a microRNA has complete sequence complementarity with a target mRNA, it instead directs cleavage of the transcript.
- X-chromosome inactivation
-
The process that occurs in female mammals by which gene expression from one of the pair of X chromosomes is downregulated to match the levels of gene expression from the single X chromosome that is present in males. The inactivation process involves a range of epigenetic mechanisms on the inactivated chromosome, including changes in DNA methylation and histone modifications.
- Genomic imprinting
-
The epigenetic marking of a locus on the basis of parental origin, which results in monoallelic gene expression.
- Epigenome
-
The global epigenetic patterns that distinguish or are variable between cell types. These patterns include DNA methylation, histone modifications and chromatin-associated proteins.
- Melanocyte
-
A specialized cell type, lying at the boundary between the dermis and epidermis, in which the pigment melanin is synthesized.
- Embryonic stem cell
-
A type of pluripotent stem cell that is derived from the inner cell mass of the early embryo. Pluripotent cells are capable of generating virtually all cell types of the organism.
- Angelman syndrome
-
A defect that is caused by the loss of expression of a maternally expressed gene, UBE3A, which is imprinted only in the brain and encodes an E3 ubiquitin ligase. Angelman syndrome occurs in ∼1 in 15,000 births and its main characteristics include mental retardation, speech impairment and behavioural abnormalities.
- Prader–Willi syndrome
-
The molecular defect that causes this syndrome is complex and involves defects that affect an ∼2 Mb imprinted domain that contains both paternally and maternally expressed genes. Prader–Willi syndrome occurs in ∼1 in 20,000 births and is characterized by a failure to thrive during infancy, hyperphagia and obesity during early childhood, mental retardation and behavioural problems.
- Beckwith–Weidemann syndrome
-
A predominantly maternally transmitted disorder, involving fetal and postnatal overgrowth and a predisposition to embryonic tumours. The Beckwith–Weidemann syndrome locus includes several imprinted genes, including IGF2, H19 and KCNQ1, and loss of imprinting at IGF2 is seen in ∼20% of cases.
- Bisulphite conversion
-
A technique that is used to identify methylcytosines. The approach depends on the relative resistance of the conversion of methylcytosine to uracil compared with cytosine. Conversion can be followed by PCR amplification and sequencing of the DNA. The persistence of a cytosine, instead of a thymine, being detected, reflects the methylation of the cytosine in the starting DNA sample.
- Machine learning
-
The ability of a program to learn from experience — that is, to modify its execution on the basis of newly acquired information. In bioinformatics, neural networks and Monte Carlo Markov chains are well-known examples.
Rights and permissions
About this article
Cite this article
Jirtle, R., Skinner, M. Environmental epigenomics and disease susceptibility. Nat Rev Genet 8, 253–262 (2007). https://doi.org/10.1038/nrg2045
Issue Date:
DOI: https://doi.org/10.1038/nrg2045