Key Points
-
The mechanisms of neuronal cell death that accompany injury and neurodegenerative disease are conserved from invertebrates to humans and, therefore, the molecular genetic dissection of these mechanisms in nematodes and flies can provide new insights into neurodegeneration.
-
Hypoxia activates a conserved response in which the oxygen sensor EGL-9 indirectly regulates the stability of the transcription factor HIFα. Invertebrate genetics have implicated ADAR RNA-editing function and part of an insulin-like signalling pathway (DAF-2 insulin like receptor, DAF-18/PTEN and DAF-16 forkhead transcription factor) in hypoxia sensitivity.
-
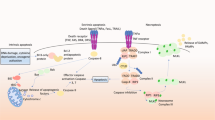
Ion-channel hyperactivation in nematodes and flies can induce necrotic cell-death in a similar way to mammalian excitotoxicity. Dissection of the mechanisms of necrosis in nematodes confirms that under conditions of excess ion influx, intracellular calcium levels rise and calpain and cathepsin proteases are activated.
-
A subset of nematode and fly models of neurodegenerative disorders involve homologues of human disease genes — for example, worm cup-5 and human mucolipidosis type IV, and fly Crumbs and human retinitis pigmentosa — or seem to affect common pathways (such as fly bubblegum and human adrenoleukodystrophy).
-
The over-expression of human disease genes in nematodes and flies has been highly successful in modelling human neurodegenerative disorders, including the pathology of Aβ1-42 (implicated in Alzheimer disease), α-synuclein (implicated in Parkinson disease), and expanded polygultamine tract proteins (implicated in several disorders including Huntington disease).
-
Processing of the human amyloid precursor protein (APP) and the Notch family of transmembrane receptors is accomplished by presenilins, which can cause human Alzheimer disease when mutated. Invertebrate genetics has helped to identify several proteins (for example, APH-2/agoraphobic/nicastrin, and APH-1, PEN-1 and SEL-10) that interact with presenilins to influence potentially toxic cleavage functions.
-
The over-expression of human α-synuclein in flies can induce relatively selective degeneration of dopaminergic neurons that include structures that resemble Lewy bodies — characteristic of human Parkinson disease. The overexpression of heat-shock chaperones HSP70 and/or HSP40, or treatment with geldanamycin (which enhances HSP70 activity) can be neuroprotective in this model.
-
The over-expression of proteins or fragments that include 'expanded' polyglutamine tracts induces neurodegeneration, which occurs in human Huntington disease, spinobulbar muscular atrophy and the spinocerebellar ataxias. Genetic suppressor screens in flies have implicated HSP70 and HSP40 as neuroprotective. Moreover, such screens have identified new death-suppressors that might be exploited for the design of therapeutics.
-
An unanticipated theme emerging from several distinct models of neurodegenerative disorders (Alzheimer disease, Parkinson disease and polyglutamine-expansion disorders) is that an increase in the activities of specific heat-shock proteins is neuroprotective. Therefore, disrupted protein folding/turnover might lie at the heart of several neurodegenerative conditions.
Abstract
If invertebrate neurons are injured by hostile environments or aberrant proteins they die much like human neurons, indicating that the powerful advantages of invertebrate molecular genetics might be successfully used for testing specific hypotheses about human neurological diseases, for drug discovery and for non-biased screens for suppressors and enhancers of neurodegeneration. Recent molecular dissection of the genetic requirements for hypoxia, excitotoxicity and death in models of Alzheimer disease, polyglutamine-expansion disorders, Parkinson disease and more, is providing mechanistic insights into neurotoxicity and suggesting new therapeutic interventions. An emerging theme is that neuronal crises of distinct origins might converge to disrupt common cellular functions, such as protein folding and turnover.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Baumeister, R. & Ge, L. The worm in us: Caenorhabditis elegans as a model of human disease. Trends Biotechnol. 20, 147–148 (2002).
Bernards, A. & Hariharan, I. K. Of flies and men — studying human disease in Drosophila. Curr. Opin. Genet. Dev. 11, 274–278 (2001).
Bargmann, C. I. Neurobiology of the Caenorhabditis elegans genome. Science 282, 2028–2033 (1998).
Yoshihara, M., Ensminger, A. W. & Littleton, J. T. Neurobiology and the Drosophila genome. Funct. Integr. Genomics 1, 235–240 (2001).
Liu, Q. A. & Hengartner, M. O. The molecular mechanism of programmed cell death in C. elegans. Ann. N Y Acad. Sci. 887, 92–104 (1999).
Richardson, H. & Kumar, S. Death to flies: Drosophila as a model system to study programmed cell death. J. Immunol. Methods 265, 21–38 (2002).
Lee, J. M., Grabb, M. C., Zipfel, G. J. & Choi, D. W. Brain tissue responses to ischemia. J. Clin. Invest. 106, 723–731 (2000).
Haddad, G. G., Sun, Y., Wyman, R. J. & Xu, T. Genetic basis of tolerance to O2 deprivation in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 94, 10809–10812 (1997).
Ma, E., Gu, X. Q., Wu, X., Xu, T. & Haddad, G. G. Mutation in pre-mRNA adenosine deaminase markedly attenuates neuronal tolerance to O2 deprivation in Drosophila melanogaster. J. Clin. Invest. 107, 685–693 (2001).
Palladino, M. J., Keegan, L. P., O'Connell, M. A. & Reenan, R. A. A-to-I pre-mRNA editing in Drosophila is primarily involved in adult nervous system function and integrity. Cell 102, 437–449 (2000).
Scott, B. A., Avidan, M. S. & Crowder, C. M. Regulation of hypoxic death in C. elegans by the insulin/IGF receptor homolog DAF-2. Science 296, 2388–2391 (2002). A genetic screen for hypoxia-resistance genes that identified roles for specific alleles of insulin-like receptor DAF-2 and some other components of the C. elegans insulin-like signalling pathway that are involved in hypoxia responses.
Gagliardi, R. J. Neuroprotection, excitotoxicity and NMDA antagonists. Arq. Neuropsiquiatr. 58, 583–588 (2000).
Rossi, D. J., Oshima, T. & Attwell, D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature 403, 316–321 (2000).
Sattler, R. & Tymianski, M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol. Neurobiol. 24, 107–129 (2001).
Heintz, N. & Zoghbi, H. Y. Insights from mouse models into the molecular basis of neurodegeneration. Annu. Rev. Physiol. 62, 779–802 (2000).
Yoon, J. et al. Novel mechanism of massive photoreceptor degeneration caused by mutations in the trp gene of Drosophila. J. Neurosci. 20, 649–659 (2000).
Treinin, M. & Chalfie, M. A mutated acetylcholine receptor subunit causes neuronal degeneration in C. elegans. Neuron 14, 871–877 (1995).
Treinin, M., Gillo, B., Liebman, L. & Chalfie, M. Two functionally dependent acetylcholine subunits are encoded in a single Caenorhabditis elegans operon. Proc. Natl Acad. Sci. USA 95, 15492–15495 (1998).
Yassin, L. et al. Characterization of the deg-3/des-2 receptor: a nicotinic acetylcholine receptor that mutates to cause neuronal degeneration. Mol. Cell. Neurosci. 17, 589–599 (2001).
Royal, D. & Driscoll, M. in Cell Death and Diseases of the Nervous System (eds Koliatsos, V. E. & Ratan, R. R.) 123–144 (Humana Press Inc., New Jersey, 1999).
Korswagen, H. C., Park, J. H., Ohshima, Y. & Plasterk, R. H. An activating mutation in a Caenorhabditis elegans Gs protein induces neural degeneration. Genes. Dev. 11, 1493–1503 (1997).
Berger, A. J., Hart, A. C. & Kaplan, J. M. Gαs-induced neurodegeneration in Caenorhabditis elegans. J. Neurosci. 18, 2871–2880 (1998).
Korswagen, H. C., van der Linden, A. M. & Plasterk, R. H. G protein hyperactivation of the Caenorhabditis elegans adenylyl cyclase SGS-1 induces neuronal degeneration. Embo J. 17, 5059–5065 (1998).
Driscoll, M. & Chalfie, M. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature 349, 588–593 (1991).
Hong, K. & Driscoll, M. A transmembrane domain of the putative channel subunit MEC-4 influences mechanotransduction and neurodegeneration in C. elegans. Nature 367, 470–473 (1994).
Adams, C. M., Snyder, P. M., Price, M. P. & Welsh, M. J. Protons activate brain Na+ channel 1 by inducing a conformational change that exposes a residue associated with neurodegeneration. J. Biol. Chem. 273, 30204–30207 (1998).
Goodman, M. B. et al. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature 415, 1039–1042 (2002).
Hall, D. H. et al. Neuropathology of degenerative cell death in Caenorhabditis elegans. J. Neurosci. 17, 1033–1045 (1997).
Schmitz, G. & Muller, G. Structure and function of lamellar bodies, lipid–protein complexes involved in storage and secretion of cellular lipids. J. Lipid Res. 32, 1539–1570 (1991).
Chung, S., Gumienny, T. L., Hengartner, M. O. & Driscoll, M. A common set of engulfment genes mediates removal of both apoptotic and necrotic cell corpses in C. elegans. Nature Cell Biol. 2, 931–937 (2000).
Syntichaki, P., Xu, K., Driscoll, M. & Tavernarakis, N. Specific aspartyl and calpain proteases are required for neurodegeneration in C. elegans. Nature 419, 939–944 (2002). This work showed that, as in humans, Ca2+-activated proteases and cathepsin proteases are required for efficient progression through necrosis in C. elegans.
Xu, K., Tavernarakis, N. & Driscoll, M. Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca2+ release from the endoplasmic reticulum. Neuron 31, 957–971 (2001). This work described identification of essential downstream components for ion channel-induced neurodegeneration in C. elegans and supported the theory that a rise in intracellular calcium is essential for necrotic cell death.
Michalak, M., Corbett, E. F., Mesaeli, N., Nakamura, K. & Opas, M. Calreticulin: one protein, one gene, many functions. Biochem. J. 344, 281–292 (1999).
Mattson, M. P. et al. Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders. Trends. Neurosci. 23, 222–229 (2000).
Chan, S. L. & Mattson, M. P. Caspase and calpain substrates: roles in synaptic plasticity and cell death. J. Neurosci. Res. 58, 167–190 (1999).
Yamashima, T. Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. Prog. Neurobiol. 62, 273–295 (2000).
Huang, Y. & Wang, K. K. The calpain family and human disease. Trends Mol. Med. 7, 355–362 (2001).
Yamashima, T. et al. Transient brain ischaemia provokes Ca2+, PIP2 and calpain responses prior to delayed neuronal death in monkeys. Eur. J. Neurosci. 8, 1932–1944 (1996).
Heisenberg, M. & Böhl, K. Isolation of anatomical brain mutants of Drosophila by histological means. Z. Naturforsch 34, 143–147 (1979).
Buchanan, R. L. & Benzer, S. Defective glia in the Drosophila brain degeneration mutant drop-dead. Neuron 10, 839–850 (1993).
Kretzschmar, D., Hasan, G., Sharma, S., Heisenberg, M. & Benzer, S. The swiss cheese mutant causes glial hyperwrapping and brain degeneration in Drosophila. J. Neurosci. 17, 7425–7432 (1997).
Min, K. T. & Benzer, S. Spongecake and eggroll: two hereditary diseases in Drosophila resemble patterns of human brain degeneration. Curr. Biol. 7, 885–888 (1997).
Min, K. T. & Benzer, S. Preventing neurodegeneration in the Drosophila mutant bubblegum. Science 284, 1985–1988 (1999). One of a series of papers that describe endogenous neurodegenerative mutations in the fly, in this case with notable resemblance to human adrenoleukodystrophy.
Palladino, M. J., Hadley, T. J. & Ganetzky, B. Temperature-sensitive paralytic mutants are enriched for those causing neurodegeneration in Drosophila. Genetics 161, 1197–1208 (2002).
Fortini, M. E., Skupski, M. P., Boguski, M. S. & Hariharan, I. K. A survey of human disease gene counterparts in the Drosophila genome. J. Cell Biol. 150, F23–F30 (2000).
Rubin, G. M. et al. Comparative genomics of the eukaryotes. Science 287, 2204–2215 (2000).
Sonnhammer, E. L. & Durbin, R. Analysis of protein domain families in Caenorhabditis elegans. Genomics 46, 200–216 (1997).
Fares, H. & Greenwald, I. Regulation of endocytosis by CUP-5, the Caenorhabditis elegans mucolipin-1 homolog. Nature Genet. 28, 64–68 (2001).
Hersh, B. M., Hartwieg, E. & Horvitz, H. R. The Caenorhabditis elegans mucolipin-like gene cup-5 is essential for viability and regulates lysosomes in multiple cell types. Proc. Natl Acad. Sci. USA 99, 4355–4360 (2002).
Izaddoost, S., Nam, S. C., Bhat, M. A., Bellen, H. J. & Choi, K. W. Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature 416, 178–183 (2002).
Johnson, K., Grawe, F., Grzeschik, N. & Knust, E. Drosophila crumbs is required to inhibit light-induced photoreceptor degeneration. Curr. Biol. 12, 1675–1680 (2002).
Pellikka, M. et al. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature 416, 143–149 (2002).
den Hollander, A. I. et al. Leber congenital amaurosis and retinitis pigmentosa with Coats-like exudative vasculopathy are associated with mutations in the crumbs homologue 1 (CRB1) gene. Am. J. Hum. Genet. 69, 198–203 (2001).
Kemp, S. et al. ABCD1 mutations and the X-linked adrenoleukodystrophy mutation database: role in diagnosis and clinical correlations. Hum. Mutat. 18, 499–515 (2001).
Mosser, J. et al. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 361, 726–730 (1993).
Raeber, A. J., Muramoto, T., Kornberg, T. B. & Prusiner, S. B. Expression and targeting of Syrian hamster prion protein induced by heat shock in transgenic Drosophila melanogaster. Mech. Dev. 51, 317–327 (1995).
Hurd, D. D. & Saxton, W. M. Kinesin mutations cause motor neuron disease phenotypes by disrupting fast axonal transport in Drosophila. Genetics 144, 1075–1085 (1996).
Elia, A. J. et al. Expression of human FALS SOD in motorneurons of Drosophila. Free Radic. Biol. Med. 26, 1332–1338 (1999).
Oeda, T. et al. Oxidative stress causes abnormal accumulation of familial amyotrophic lateral sclerosis-related mutant SOD1 in transgenic Caenorhabditis elegans. Hum. Mol. Genet. 10, 2013–2023 (2001).
Selkoe, D. J. Presenilin, Notch, and the genesis and treatment of Alzheimer's disease. Proc. Natl Acad. Sci. USA 98, 11039–11041 (2001).
Daigle, I. & Li, C. apl-1, a Caenorhabditis elegans gene encoding a protein related to the human β-amyloid protein precursor. Proc. Natl Acad. Sci. USA 90, 12045–12049 (1993).
Rosen, D. R., Martin-Morris, L., Luo, L. Q. & White, K. A Drosophila gene encoding a protein resembling the human β-amyloid protein precursor. Proc. Natl Acad. Sci. USA 86, 2478–2482 (1989).
Luo, L., Tully, T. & White, K. Human amyloid precursor protein ameliorates behavioral deficit of flies deleted for Appl gene. Neuron 9, 595–605 (1992).
Torroja, L., Packard, M., Gorczyca, M., White, K. & Budnik, V. The Drosophila β-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. J. Neurosci. 19, 7793–7803 (1999).
Gunawardena, S. & Goldstein, L. S. Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in Drosophila. Neuron 32, 389–401 (2001).
Link, C. D. Expression of human β-amyloid peptide in transgenic Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 92, 9368–9372 (1995).
Fay, D. S., Fluet, A., Johnson, C. J. & Link, C. D. In vivo aggregation of β-amyloid peptide variants. J. Neurochem. 71, 1616–1625 (1998).
Tabira, T., Chui, D. H. & Kuroda, S. Significance of intracellular Aβ42 accumulation in Alzheimer's disease. Front. Biosci. 7, a44–a49 (2002).
Glabe, C. Intracellular mechanisms of amyloid accumulation and pathogenesis in Alzheimer's disease. J. Mol. Neurosci. 17, 137–145 (2001).
Link, C. D. et al. Visualization of fibrillar amyloid deposits in living, transgenic Caenorhabditis elegans animals using the sensitive amyloid dye, X-34. Neurobiol. Aging 22, 217–226 (2001).
Fonte, V. et al. Interaction of intracellular β amyloid peptide with chaperone proteins. Proc. Natl Acad. Sci. USA 99, 9439–9444 (2002). This paper implicates specific heat-shock chaperones in toxicity and metabolism of the intracellular Alzheimer disease-causing Aβ 1-42 fragment in nematodes.
Selkoe, D. J. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 81, 741–766 (2001).
Levitan, D. & Greenwald, I. Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer's disease gene. Nature 377, 351–354 (1995).
Li, X. & Greenwald, I. HOP-1, a Caenorhabditis elegans presenilin, appears to be functionally redundant with SEL-12 presenilin and to facilitate LIN-12 and GLP-1 signaling. Proc. Natl Acad. Sci. USA 94, 12204–12209 (1997).
Arduengo, P. M., Appleberry, O. K., Chuang, P. & L'Hernault, S. W. The presenilin protein family member SPE-4 localizes to an ER/Golgi derived organelle and is required for proper cytoplasmic partitioning during Caenorhabditis elegans spermatogenesis. J. Cell. Sci. 111, 3645–3654 (1998).
Boulianne, G. L. et al. Cloning and characterization of the Drosophila presenilin homologue. Neuroreport 8, 1025–1029 (1997).
Hong, C. S. & Koo, E. H. Isolation and characterization of Drosophila presenilin homolog. Neuroreport 8, 665–668 (1997).
Ye, Y., Lukinova, N. & Fortini, M. E. Neurogenic phenotypes and altered Notch processing in Drosophila presenilin mutants. Nature 398, 525–529 (1999). One of the first publications to show the activity of presenilin in Notch processing in the fly.
Okochi, M. et al. A loss of function mutant of the presenilin homologue SEL-12 undergoes aberrant endoproteolysis in Caenorhabditis elegans and increases aβ 42 generation in human cells. J. Biol. Chem. 275, 40925–40932 (2000).
Goutte, C., Hepler, W., Mickey, K. M. & Priess, J. R. aph-2 encodes a novel extracellular protein required for GLP-1-mediated signaling. Dev. Suppl. 127, 2481–2492 (2000).
Yu, G. et al. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and βAPP processing. Nature 407, 48–54 (2000).
Chung, H. M. & Struhl, G. Nicastrin is required for presenilin-mediated transmembrane cleavage in Drosophila. Nature Cell Biol. 3, 1129–1132 (2001).
Lopez-Schier, H. & St Johnston, D. Drosophila nicastrin is essential for the intramembranous cleavage of notch. Dev. Cell 2, 79–89 (2002).
Goutte, C., Tsunozaki, M., Hale, V. A. & Priess, J. R. APH-1 is a multipass membrane protein essential for the Notch signaling pathway in Caenorhabditis elegans embryos Proc. Natl Acad. Sci. USA 99, 775–779 (2002).
Francis, R. et al. aph-1 and pen-2 are required for Notch pathway signaling, γ-secretase cleavage of βAPP, and presenilin protein accumulation. Dev. Cell 3, 85–97 (2002). A report of a genetic-interaction screen that identified two C. elegans proteins (with functional human homologues) as new components of the presenilin γ-secretase complex.
Hubbard, E. J., Wu, G., Kitajewski, J. & Greenwald, I. sel-10, a negative regulator of lin-12 activity in Caenorhabditis elegans, encodes a member of the CDC4 family of proteins. Genes Dev. 11, 3182–3193 (1997).
Wu, G., Hubbard, E. J., Kitajewski, J. K. & Greenwald, I. Evidence for functional and physical association between Caenorhabditis elegans SEL-10, a Cdc4p-related protein, and SEL-12 presenilin. Proc. Natl Acad. Sci. USA 95, 15787–15791 (1998).
Li, J. et al. SEL-10 interacts with presenilin 1, facilitates its ubiquitylation, and alters A-β peptide production. J. Neurochem. 82, 1540–1548 (2002).
Wittenburg, N. et al. Presenilin is required for proper morphology and function of neurons in C. elegans. Nature 406, 306–309 (2000). This study shows a role for presenilin in neuronal morphology and function and specifically implicates presenilin function in thermal memory of C. elegans.
Giasson, B. I. & Lee, V. M. Parkin and the molecular pathways of Parkinson's disease. Neuron 31, 885–888 (2001).
Duda, J. E., Lee, V. M. & Trojanowski, J. Q. Neuropathology of synuclein aggregates. J. Neurosci. Res. 61, 121–127 (2000).
Schwartz, L. M., Nambu, J. R. & Wang, Z. Parkinsonism proteolysis and proteasomes. Cell Death Differ. 9, 479–482 (2002).
Nass, R., Miller, D. M. & Blakely, R. D. C. elegans: a novel pharmacogenetic model to study Parkinson's disease. Parkinsonism Relat. Disord. 7, 185–191 (2001).
Nass, R., Hall, D. H., Miller, D. M. & Blakely, R. D. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 99, 3264–3269 (2002).
Feany, M. B. & Bender, W. W. A Drosophila model of Parkinson's disease. Nature 404, 394–398 (2000). The pioneering work establishing a transgenic Drosophila model for Parkinson disease in which human α-synuclein is over-expressed. The fly model features some selective vulnerability for dopaminergic neurons, formation of Lewy-like neuronal inclusions and late-onset neurodegeneration.
Auluck, P. K., Chan, H. Y., Trojanowski, J. Q., Lee, V. M. & Bonini, N. M. Chaperone suppression of α-synuclein toxicity in a Drosophila model for Parkinson's disease. Science 295, 865–868 (2002). This work shows that transgenic expression of specific HSP70- and HSP40-family members can exert neuroprotective effects against α-synuclein toxicity in flies.
Auluck, P. K. & Bonini, N. M. Pharmacological prevention of Parkinson disease in Drosophila. Nature Med. 8, 1185–1186 (2002). This paper reports that geldanamycin treatment, which potentiates HSP70 activity, can be neuroprotective against over-expression of α-synuclein, a good example of how flies can be used to test working hypotheses and potential pharmacological intervention strategies.
Pendleton, R. G., Parvez, F., Sayed, M. & Hillman, R. Effects of pharmacological agents upon a transgenic model of Parkinson's disease in Drosophila melanogaster. J. Pharmacol. Exp. Ther. 300, 91–96 (2002).
Zoghbi, H. Y. & Botas, J. Mouse and fly models of neurodegeneration. Trends Genet. 18, 463–471 (2002).
Zoghbi, H. Y. & Orr, H. T. Glutamine repeats and neurodegeneration. Annu. Rev. Neurosci. 23, 217–247 (2000).
Warrick, J. M. et al. Expanded polyglutamine protein forms nuclear inclusions and causes neural degeneration in Drosophila. Cell 93, 939–949 (1998). One of several examples of invertebrate polyglutamine-expansion disease models that parallel human disease progression and cellular characteristics. It is also the basis of a genetic screen described in reference 109.
Jackson, G. R. et al. Polyglutamine-expanded human huntingtin transgenes induce degeneration of Drosophila photoreceptor neurons. Neuron 21, 633–642 (1998).
Fernandez-Funez, P. et al. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature 408, 101–106 (2000). A genetic screen that identified proteins with a range of biological functions as modulators of neuronal degeneration caused by expression of an expanded polyglutamine protein.
Chan, H. Y., Warrick, J. M., Gray-Board, G. L., Paulson, H. L. & Bonini, N. M. Mechanisms of chaperone suppression of polyglutamine disease: selectivity, synergy and modulation of protein solubility in Drosophila. Hum. Mol. Genet. 9, 2811–2820 (2000).
Takeyama, K. et al. Androgen-dependent neurodegeneration by polyglutamine-expanded human androgen receptor in Drosophila. Neuron 35, 855–864 (2002).
Marsh, J. L. et al. Expanded polyglutamine peptides alone are intrinsically cytotoxic and cause neurodegeneration in Drosophila. Hum. Mol. Genet. 9, 13–25 (2000).
Kazemi-Esfarjani, P. & Benzer, S. Genetic suppression of polyglutamine toxicity in Drosophila. Science 287, 1837–1840 (2000). This work describes a genetic screen that showed several loci (including two heat-shock chaperone genes) that enhance or suppress neurodegeneration induced by expression of an expanded polyglutamine fragment.
Faber, P. W., Alter, J. R., MacDonald, M. E. & Hart, A. C. Polyglutamine-mediated dysfunction and apoptotic death of a Caenorhabditis elegans sensory neuron. Proc. Natl Acad. Sci. USA 96, 179–184 (1999).
Warrick, J. M. et al. Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nature Genet. 23, 425–428 (1999). A demonstration that HSP70 over-expression can suppress neurodegeneration in a fly model of spinocerebellar ataxia (MJD/SCA-3). A similar finding was later reported for a mouse SCA-1 model (reference 114).
Satyal, S. H. et al. Polyglutamine aggregates alter protein folding homeostasis in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 97, 5750–5755 (2000).
Kazantsev, A. et al. A bivalent Huntingtin binding peptide suppresses polyglutamine aggregation and pathogenesis in Drosophila. Nature Genet. 30, 367–376 (2002).
Steffan, J. S. et al. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 413, 739–743 (2001).
Morley, J. F., Brignull, H. R., Weyers, J. J. & Morimoto, R. I. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 99, 10417–10422 (2002).
Cummings, C. J. et al. Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum. Mol. Genet. 10, 1511–1518 (2001).
Ross, C. Polyglutamine pathogenesis: emergence of unifying mechanisms for Huntington's disease and related disorders. Neuron 35, 819 (2002).
Holbert, S. et al. The Gln-Ala repeat transcriptional activator CA150 interacts with huntingtin: neuropathologic and genetic evidence for a role in Huntington's disease pathogenesis. Proc. Natl Acad. Sci. USA 98, 1811–1816 (2001).
Faber, P. W., Voisine, C., King, D. C., Bates, E. A. & Hart, A. C. Glutamine/proline-rich PQE-1 proteins protect Caenorhabditis elegans neurons from huntingtin polyglutamine neurotoxicity. Proc. Natl Acad. Sci. USA 99, 17131–17136 (2002). A description of a new modifier of polyglutamine-expansion-induced neurodegeneration.
Nambu, J. R., Chen, W., Hu, S. & Crews, S. T. The Drosophila melanogaster similar bHLH-PAS gene encodes a protein related to human hypoxia-inducible factor 1 α and Drosophila single-minded. Gene 172, 249–254 (1996).
Jiang, H., Guo, R. & Powell-Coffman, J. A. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc. Natl Acad. Sci. USA 98, 7916–7921 (2001).
Semenza, G. L. HIF-1 and human disease: one highly involved factor. Genes Dev. 14, 1983–1991 (2000).
Epstein, A. C. et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 (2001).
Lavista-Llanos, S. et al. Control of the hypoxic response in Drosophila melanogaster by the basic helix-loop-helix PAS protein Similar. Mol. Cell. Biol. 22, 6842–6853 (2002).
Wingrove, J. A. & O'Farrell, P. H. Nitric oxide contributes to behavioral, cellular, and developmental responses to low oxygen in Drosophila. Cell 98, 105–114 (1999).
Stamler, J. S. et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 276, 2034–2037 (1997).
Xiong, W. C., Okano, H., Patel, N. H., Blendy, J. A. & Montell, C. repo encodes a glial-specific homeo domain protein required in the Drosophila nervous system. Genes Dev. 8, 981–994 (1994).
Xiong, W. C. & Montell, C. Defective glia induce neuronal apoptosis in the repo visual system of Drosophila. Neuron 14, 581–590 (1995).
Nakano, Y. et al. Mutations in the novel membrane protein spinster interfere with programmed cell death and cause neural degeneration in Drosophila melanogaster. Mol. Cell Biol. 21, 3775–3788 (2001).
Min, K. Drosophila as a model to study human brain degenerative diseases. Parkinsonism Relat. Disord. 7, 165–169 (2001).
Fortini, M. E. γ-secretase-mediated proteolysis in cell-surface-receptor signalling. Nature Rev. Mol. Cell. Biol. 3, 673–684 (2002).
Selkoe, D. J. Translating cell biology into therapeutic advances in Alzheimer's disease. Nature 399, A23–A31 (1999).
Struhl, G. & Greenwald, I. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature 398, 522–525 (1999).
DiFiglia, M. et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277, 1990–1993 (1997).
Vonsattel, J. P. et al. Neuropathological classification of Huntington's disease. J. Neuropathol. Exp. Neurol. 44, 559–577 (1985).
Parker, J. A. et al. Expanded polyglutamines in Caenorhabditis elegans cause axonal abnormalities and severe dysfunction of PLM mechanosensory neurons without cell death. Proc. Natl Acad. Sci. USA 98, 13318–13323 (2001).
Chan, H. Y., Warrick, J. M., Andriola, I., Merry, D. & Bonini, N. M. Genetic modulation of polyglutamine toxicity by protein conjugation pathways in Drosophila. Hum. Mol. Genet. 11, 2895–2904 (2002).
Schenk, D. amyloid-β immunotherapy for Alzheimer's disease: the end of the beginning. Nature Rev. Neurosci. 3, 824–828 (2002).
Acknowledgements
We thank I. Mano and G. Stamatas for critical reading of the manuscript. B.G. is a Fullbright grantee and was supported by a Louis Bevier Graduate Fellowship. Work was supported in part by grants from the National Institutes of Health/National Institute of Neurological Disorders and Stroke, and the National Institute on Ageing.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Related links
Related links
DATABASES
LocusLink
OMIM
Autosomal recessive juvenile-onset parkinsonism
dentatorubral pallidoluysian atrophy
Swiss-Prot
WormBase
Glossary
- RNA INTERFERENCE
-
(RNAi). A form of post-transcriptional gene silencing, in which double-stranded RNA induces degradation of the homologous endogenous transcripts, mimicking the effect of the reduction, or loss, of gene activity.
- UBIQUITYLATION
-
A regulated process by which ubiquitin residues are conjugated to influence transport (mono-ubiquitylation) or to mark proteins for degradation (poly-ubiquitylation).
- PROTEASOME
-
A cytosolic protein complex that degrades proteins that have been marked for destruction by the ubiquitylation pathway.
- DAUER
-
An alternative developmental larval stage that worms enter when food is lacking, the population is crowded or growth temperatures are suboptimal.
- DESENSITIZATION
-
Adaptive changes in ion-channel signalling that limit the responses to continuous receptor stimulation.
- MULTILAMELLAR INCLUSIONS
-
Electron-dense lipid-containing subcellular structures — which often appear as concentric layers — that are observed in lipid-storage diseases and other neurodegenerative conditions.
- ISCHAEMIA
-
The reduction of oxygen levels (hypoxia) in tissues due to an inadequate blood supply.
- EXCITOTOXIC CELL DEATH
-
Neuronal death caused by the hyperstimulation of glutamate-gated ion channels (for example, as a consequence of the depletion of ATP stores under ischaemic conditions) and the consequent toxic rise in intracellular Ca2+.
- CATHEPSINS
-
A group of aspartyl proteases localized primarily to lysosomes that are implicated in necrosis.
- OPTIC LOBE
-
The part of the adult fly brain that processes sensory information from the compound eye.
- TAOPATHY
-
A group of disorders associated with abnormal accumulation of hyper-phosphorylated microtubule binding protein tau in intracellular neurofibrillary tangles.
- AMYLOID PLAQUES
-
Extracellular, insoluble aggregations of Aβ1-42-fragment, cleaved from the amyloid precursor protein (APP), that accumulate in the brains of Alzheimer disease patients.
- PEROXISOME
-
An organelle that contains machinery to oxidize organic compounds. The site of H202 production and destruction.
- LIMBIC CORTEX
-
The regions of the brain that are associated with emotion.
- ASSOCIATION CORTEX
-
The regions of the brain that are involved in the processing and integration of sensory and sensorimotor information.
- GLIAL CELLS
-
The specialized cells of the central nervous system that are associated with neurons and provide mechanical support, supply nutrients, oxygen and growth factors, and eliminate potentially harmful metabolites and neurotransmitters.
- BRADYKINESIA
-
Slow movement — a symptom associated with Parkinson disease.
- DYSTONIA
-
A disease associated with muscle spasms and twisting of the arms and head.
- CHOREA
-
Involuntary jerky movements of the head, face or limbs, which are characteristic of Huntington disease.
- PHOTOTAXIS
-
Movement towards a light source. Phototaxis assays test locomotor activity in flies.
- SUBSTANTIA NIGRA
-
(SN). The region of the brain that includes the pars compacta, harbouring the neurons that produce the neurotransmitter dopamine required for controlled locomotion. SN neurons degenerate in Parkinson disease.
- LOCUS CERULEUS
-
The area of the brainstem that undergoes degeneration in Parkinson disease.
- LEWY STRUCTURES
-
The intracellular inclusions that function as histological markers for degenerating neurons in the brains of individuals with certain neurodegenerative disorders, including Parkinson disease.
- DOPAMINERGIC
-
Neurons that use dopamine as a neurotransmitter.
- PROTOFILAMENTS
-
The ordered insoluble structures formed by Aβ fragments. In Alzheimer disease, Aβ fragments dimerize and then multimerize into antiparallel β-sheets that form helical protofilaments.
Rights and permissions
About this article
Cite this article
Driscoll, M., Gerstbrein, B. Dying for a cause: invertebrate genetics takes on human neurodegeneration. Nat Rev Genet 4, 181–194 (2003). https://doi.org/10.1038/nrg1018
Issue Date:
DOI: https://doi.org/10.1038/nrg1018