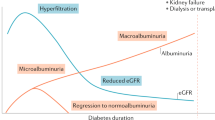
Abstract
Over the past three decades, the number of people with diabetes mellitus has more than doubled globally, making it one of the most important public health challenges to all nations. Type 2 diabetes mellitus (T2DM) and prediabetes are increasingly observed among children, adolescents and younger adults. The causes of the epidemic of T2DM are embedded in a very complex group of genetic and epigenetic systems interacting within an equally complex societal framework that determines behavior and environmental influences. This complexity is reflected in the diverse topics discussed in this Review. In the past few years considerable emphasis has been placed on the effect of the intrauterine environment in the epidemic of T2DM, particularly in the early onset of T2DM and obesity. Prevention of T2DM is a 'whole-of-life' task and requires an integrated approach operating from the origin of the disease. Future research is necessary to better understand the potential role of remaining factors, such as genetic predisposition and maternal environment, to help shape prevention programs. The potential effect on global diabetes surveillance of using HbA1c rather than glucose values in the diagnosis of T2DM is also discussed.
Key Points
-
The prevalence of type 2 diabetes mellitus (T2DM) and prediabetes has been rapidly rising worldwide over the past three decades, particularly in developing countries
-
In addition to the early onset of T2DM in young adults, an increasing trend of T2DM and prediabetes is noticeable among children and adolescents
-
The epidemic of T2DM is attributable to a mixture of genetic and epigenetic predispositions and a variety of behavioral and environmental risk factors
-
An integrated approach, taking into account genetic and epigenetic determinants, is required for the effective prevention of T2DM beginning from the start of life
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Zimmet, P., Alberti, K. G. & Shaw, J. Global and societal implications of the diabetes epidemic. Nature 414, 782–787 (2001).
Danaei, G. et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 378, 31–40 (2011).
Shaw, J. E., Sicree, R. A. & Zimmet, P. Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 87, 4–14 (2010).
Chan, J. C. et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 301, 2129–2140 (2009).
Dowse, G. K. et al. High prevalence of NIDDM and impaired glucose tolerance in Indian, Creole, and Chinese Mauritians. Mauritius Noncommunicable Disease Study Group. Diabetes 39, 390–396 (1990).
Simmons, D., Williams, D. R. & Powell, M. J. Prevalence of diabetes in a predominantly Asian community: preliminary findings of the Coventry diabetes study. BMJ 298, 18–21 (1989).
McNeely, M. J. & Boyko, E. J. Type 2 diabetes prevalence in Asian Americans: results of a national health survey. Diabetes Care 27, 66–69 (2004).
Yang, W. et al. Prevalence of diabetes among men and women in China. N. Engl. J. Med. 362, 1090–1101 (2010).
Mbanya, J. C., Motala, A. A., Sobngwi, E., Assah, F. K. & Enoru, S. T. Diabetes in sub-Saharan Africa. Lancet 375, 2254–2266 (2010).
Abubakari, A. R. et al. Prevalence and time trends in diabetes and physical inactivity among adult West African populations: the epidemic has arrived. Public Health 123, 602–614 (2009).
Wändell, P. E. et al. Estimation of diabetes prevalence among immigrants from the Middle East in Sweden by using three different data sources. Diabetes Metab. 34, 328–333 (2008).
Ramachandran, A., Mary, S., Yamuna, A., Murugesan, N. & Snehalatha, C. High prevalence of diabetes and cardiovascular risk factors associated with urbanization in India. Diabetes Care 31, 893–898 (2008).
Ning, F. et al. Risk factors associated with the dramatic increase in the prevalence of diabetes in the adult Chinese population in Qingdao, China. Diabet. Med. 26, 855–863 (2009).
Pinhas-Hamiel, O. & Zeitler, P. The global spread of type 2 diabetes mellitus in children and adolescents. J. Pediatr. 146, 693–700 (2005).
Liese, A. D. et al. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics 118, 1510–1518 (2006).
Kitagawa, T., Owada, M., Urakami, T. & Yamauchi, K. Increased incidence of non-insulin dependent diabetes mellitus among Japanese schoolchildren correlates with an increased intake of animal protein and fat. Clin. Pediatr. (Phila.) 37, 111–115 (1998).
Dabelea, D. et al. Incidence of diabetes in youth in the United States. JAMA 297, 2716–2724 (2007).
Craig, M. E., Femia, G., Broyda, V., Lloyd, M. & Howard, N. J. Type 2 diabetes in Indigenous and non-Indigenous children and adolescents in New South Wales. Med. J. Aust. 186, 497–499 (2007).
Dabelea, D. et al. Association testing of TCF7L2 polymorphisms with type 2 diabetes in multi-ethnic youth. Diabetologia 54, 535–539 (2011).
Williams, D. E. et al. Prevalence of impaired fasting glucose and its relationship with cardiovascular disease risk factors in US adolescents, 1999–2000. Pediatrics 116, 1122–1126 (2005).
Li, C., Ford, E. S., Zhao, G. & Mokdad, A. H. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005–2006. Diabetes Care 32, 342–347 (2009).
Sinha, R. et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N. Engl. J. Med. 346, 802–810 (2002).
Goran, M. I. et al. Impaired glucose tolerance and reduced beta-cell function in overweight Latino children with a positive family history for type 2 diabetes. J. Clin. Endocrinol. Metab. 89, 207–212 (2004).
Rokholm, B., Baker, J. L. & Sorensen, T. I. The levelling off of the obesity epidemic since the year 1999—a review of evidence and perspectives. Obes. Rev. 11, 835–846 (2010).
Mokdad, A. H. et al. Diabetes trends in the U.S.: 1990–1998. Diabetes Care 23, 1278–1283 (2000).
Lawrence, J. M. et al. Diabetes in Hispanic American youth: prevalence, incidence, demographics, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care 32 (Suppl. 2), S123–S132 (2009).
Kelly, T., Yang, W., Chen, C. S., Reynolds, K. & He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. (Lond.) 32, 1431–1437 (2008).
Hu, F. B. et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 345, 790–797 (2001).
Narayan, K. M., Boyle, J. P., Thompson, T. J., Gregg, E. W. & Williamson, D. F. Effect of BMI on lifetime risk for diabetes in the U. S. Diabetes Care 30, 1562–1566 (2007).
Schienkiewitz, A., Schulze, M. B., Hoffmann, K., Kroke, A. & Boeing, H. Body mass index history and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am. J. Clin. Nutr. 84, 427–433 (2006).
Ruderman, N., Chisholm, D., Pi-Sunyer, X. & Schneider, S. The metabolically obese, normal-weight individual revisited. Diabetes 47, 699–713 (1998).
Meigs, J. B. et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J. Clin. Endocrinol. Metab. 91, 2906–2912 (2006).
Arnlöv, J., Sundström, J., Ingelsson, E. & Lind, L. Impact of BMI and the metabolic syndrome on the risk of diabetes in middle-aged men. Diabetes Care 34, 61–65 (2011).
Yoon, K.-H. et al. Epidemic obesity and type 2 diabetes in Asia. Lancet 368, 1681–1688 (2006).
Huxley, R. et al. Ethnic comparisons of the cross-sectional relationships between measures of body size with diabetes and hypertension. Obes. Rev. 9 (Suppl. 1), 53–61 (2008).
Deurenberg, P., Deurenberg-Yap, M. & Guricci, S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes. Rev. 3, 141–146 (2002).
Kadowaki, T. et al. Japanese men have larger areas of visceral adipose tissue than Caucasian men in the same levels of waist circumference in a population-based study. Int. J. Obes. (Lond.) 30, 1163–1165 (2006).
Lear, S. A. et al. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). Am. J. Clin. Nutr. 86, 353–359 (2007).
Lebovitz, H. E. & Banerji, M. A. Point: visceral adiposity is causally related to insulin resistance. Diabetes Care 28, 2322–2325 (2005).
Perseghin, G. Lipids in the wrong place: visceral fat and nonalcoholic steatohepatitis. Diabetes Care 34 (Suppl. 2), S367–S370 (2011).
Hwang, J. H. et al. Increased intrahepatic triglyceride is associated with peripheral insulin resistance: in vivo MR imaging and spectroscopy studies. Am. J. Physiol. Endocrinol. Metab. 293, E1663–E1669 (2007).
Fraser, A. et al. Alanine aminotransferase, γ-glutamyltransferase, and incident diabetes: the British Women's Heart and Health Study and meta-analysis. Diabetes Care 32, 741–750 (2009).
Taylor, R. Pathogenesis of type 2 diabetes: tracing the reverse route from cure to cause. Diabetologia 51, 1781–1789 (2008).
Bird, A. Perceptions of epigenetics. Nature 447, 396–398 (2007).
Gluckman, P. D., Hanson, M. A., Cooper, C. & Thornburg, K. L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359, 61–73 (2008).
Pinney, S. E. & Simmons, R. A. Epigenetic mechanisms in the development of type 2 diabetes. Trends Endocrinol. Metab. 21, 223–229 (2010).
Whincup, P. H. et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA 300, 2886–2897 (2008).
Hales, C. N. & Barker, D. J. The thrifty phenotype hypothesis. Br. Med. Bull. 60, 5–20 (2001).
Ravelli, A. C. et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet 351, 173–177 (1998).
Li, Y. et al. Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes 59, 2400–2406 (2010).
Dabelea, D. et al. Birth weight, type 2 diabetes, and insulin resistance in Pima Indian children and young adults. Diabetes Care 22, 944–950 (1999).
Bavdekar, A. et al. Insulin resistance syndrome in 8-year-old Indian children: small at birth, big at 8 years, or both? Diabetes 48, 2422–2429 (1999).
Fowden, A. L. & Hill, D. J. Intra-uterine programming of the endocrine pancreas. Br. Med. Bull. 60, 123–142 (2001).
Hyppönen, E., Power, C. & Smith, G. D. Prenatal growth, BMI, and risk of type 2 diabetes by early midlife. Diabetes Care 26, 2512–2517 (2003).
King, H. et al. Diabetes and associated disorders in Cambodia: two epidemiological surveys. Lancet 366, 1633–1639 (2005).
Wei, J. N. et al. Low birth weight and high birth weight infants are both at an increased risk to have type 2 diabetes among schoolchildren in Taiwan. Diabetes Care 26, 343–348 (2003).
Al Salmi, I. et al. Disorders of glucose regulation in adults and birth weight: results from the Australian Diabetes, Obesity and Lifestyle (AUSDIAB) Study. Diabetes Care 31, 159–164 (2008).
Bellamy, L., Casas, J. P., Hingorani, A. D. & Williams, D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 373, 1773–1779 (2009).
Pettitt, D. J. et al. Congenital susceptibility to NIDDM. Role of intrauterine environment. Diabetes 37, 622–628 (1988).
Dabelea, D. et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 49, 2208–2211 (2000).
Stride, A. et al. Intrauterine hyperglycemia is associated with an earlier diagnosis of diabetes in HNF-1alpha gene mutation carriers. Diabetes Care 25, 2287–2291 (2002).
Sobngwi, E. et al. Effect of a diabetic environment in utero on predisposition to type 2 diabetes. Lancet 361, 1861–1865 (2003).
Crume, T. L. et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia 54, 87–92 (2011).
Pettitt, D. J. et al. Association between maternal diabetes in utero and age at offspring's diagnosis of type 2 diabetes. Diabetes Care 31, 2126–2130 (2008).
Franks, P. W. et al. Gestational glucose tolerance and risk of type 2 diabetes in young Pima Indian offspring. Diabetes 55, 460–465 (2006).
Lawrence, J. M., Contreras, R., Chen, W. & Sacks, D. A. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care 31, 899–904 (2008).
Dabelea, D. et al. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care 28, 579–584 (2005).
Getahun, D., Nath, C., Ananth, C. V., Chavez, M. R. & Smulian, J. C. Gestational diabetes in the United States: temporal trends 1989 through 2004. Am. J. Obstet. Gynecol. 198, 525.e1–525.e5 (2008).
Cauchi, S. et al. TCF7L2 is reproducibly associated with type 2 diabetes in various ethnic groups: a global meta-analysis. J. Mol. Med. 85, 777–782 (2007).
McCarthy, M. I. Genomics, type 2 diabetes, and obesity. N. Engl. J. Med. 363, 2339–2350 (2010).
Balkau, B. et al. Predicting diabetes: clinical, biological, and genetic approaches: data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 31, 2056–2061 (2008).
Schulze, M. B. et al. Use of multiple metabolic and genetic markers to improve the prediction of type 2 diabetes: the EPIC-Potsdam Study. Diabetes Care 32, 2116–2119 (2009).
Lango, H. et al. Assessing the combined impact of 18 common genetic variants of modest effect sizes on type 2 diabetes risk. Diabetes 57, 3129–3135 (2008).
Lyssenko, V. et al. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N. Engl. J. Med. 359, 2220–2232 (2008).
Meigs, J. B. et al. Genotype score in addition to common risk factors for prediction of type 2 diabetes. N. Engl. J. Med. 359, 2208–2219 (2008).
van Hoek, M. et al. Predicting type 2 diabetes based on polymorphisms from genome-wide association studies: a population-based study. Diabetes 57, 3122–3128 (2008).
Talmud, P. J. et al. Utility of genetic and non-genetic risk factors in prediction of type 2 diabetes: Whitehall II prospective cohort study. BMJ 340, b4838 (2010).
de Miguel-Yanes, J. M. et al. Genetic risk reclassification for type 2 diabetes by age below or above 50 years using 40 type 2 diabetes risk single nucleotide polymorphisms. Diabetes Care 34, 121–125 (2011).
Qi, L., Cornelis, M. C., Zhang, C., van Dam, R. M. & Hu, F. B. Genetic predisposition, Western dietary pattern, and the risk of type 2 diabetes in men. Am. J. Clin. Nutr. 89, 1453–1458 (2009).
Laaksonen, D. E. et al. Physical activity, diet, and incident diabetes in relation to an ADRA2B polymorphism. Med. Sci. Sports. Exerc. 39, 227–232 (2007).
Florez, J. C. et al. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N. Engl. J. Med. 355, 241–250 (2006).
Wang, J. et al. Variants of transcription factor 7-like 2 (TCF7L2) gene predict conversion to type 2 diabetes in the Finnish Diabetes Prevention Study and are associated with impaired glucose regulation and impaired insulin secretion. Diabetologia 50, 1192–1200 (2007).
Grant, R. W. et al. The clinical application of genetic testing in type 2 diabetes: a patient and physician survey. Diabetologia 52, 2299–2305 (2009).
Markowitz, S. M., Park, E. R., Delahanty, L. M., O'Brien, K. E. & Grant, R. W. Perceived impact of diabetes genetic risk testing among patients at high phenotypic risk for type 2 diabetes. Diabetes Care 34, 568–573 (2011).
Shaw, J. E., Punjabi, N. M., Wilding, J. P., Alberti, K. G. & Zimmet, P. Z. Sleep-disordered breathing and type 2 diabetes: a report from the International Diabetes Federation Taskforce on Epidemiology and Prevention. Diabetes Res. Clin. Pract. 81, 2–12 (2008).
Mezuk, B., Eaton, W. W., Albrecht, S. & Golden, S. H. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 31, 2383–2390 (2008).
Kivimäki, M. et al. Antidepressant medication use, weight gain, and risk of type 2 diabetes: a population-based study. Diabetes Care 33, 2611–2616 (2010).
Alonso-Magdalena, P., Quesada, I. & Nadal, A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 7, 346–353 (2011).
Brook, R. D. et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121, 2331–2378 (2010).
Krämer, U. et al. Traffic-related air pollution and incident type 2 diabetes: results from the SALIA cohort study. Environ. Health Perspect. 118, 1273–1279 (2010).
Nathan, D. M., Turgeon, H. & Regan, S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia 50, 2239–2244 (2007).
American Diabetes Association. Standards of medical care in diabetes–2010. Diabetes Care 33 (Suppl. 1), S11–S61 (2010).
World Health Organization. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: abbreviated report of a WHO consultation [online], (2011).
Zhang, X. et al. A1C level and future risk of diabetes: a systematic review. Diabetes Care 33, 1665–1673 (2010).
Selvin, E. et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N. Engl. J. Med. 362, 800–811 (2010).
Santos-Oliveira, R. et al. Haemoglobin A(1c) levels and subsequent cardiovascular disease in persons without diabetes: a meta-analysis of prospective cohorts. Diabetologia 54, 1327–1334 (2011).
The International Expert Committe. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32, 1327–1334 (2009).
Colagiuri, S. et al. Glycemic thresholds for diabetes-specific retinopathy: implications for diagnostic criteria for diabetes. Diabetes Care 34, 145–150 (2011).
Zhou, X. et al. Performance of an A1C and fasting capillary blood glucose test for screening newly diagnosed diabetes and pre-diabetes defined by an oral glucose tolerance test in Qingdao, China. Diabetes Care 33, 545–550 (2010).
Christensen, D. L. et al. Moving to an A1C-based diagnosis of diabetes has a different impact on prevalence in different ethnic groups. Diabetes Care 33, 580–582 (2010).
Cowie, C. C. et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care 33, 562–568 (2010).
van 't Riet, E. et al. Relationship between A1C and glucose levels in the general Dutch population: the new Hoorn study. Diabetes Care 33, 61–66 (2010).
Araneta, M. R., Grandinetti, A. & Chang, H. K. A1C and diabetes diagnosis among Filipino Americans, Japanese Americans, and Native Hawaiians. Diabetes Care 33, 2626–2628 (2010).
Olson, D. E. et al. Screening for diabetes and pre-diabetes with proposed A1C-based diagnostic criteria. Diabetes Care 33, 2184–2189 (2010).
Boronat, M. et al. Differences in cardiovascular risk profile of diabetic subjects discordantly classified by diagnostic criteria based on glycated hemoglobin and oral glucose tolerance test. Diabetes Care 33, 2671–2673 (2010).
Mostafa, S. A. et al. The potential impact of using glycated haemoglobin as the preferred diagnostic tool for detecting type 2 diabetes mellitus. Diabet. Med. 27, 762–769 (2010).
Bao, Y. et al. Glycated haemoglobin A1c for diagnosing diabetes in Chinese population: cross sectional epidemiological survey. BMJ 340, c2249 (2010).
Selvin, E., Zhu, H. & Brancati, F. L. Elevated A1C in adults without a history of diabetes in the U.S. Diabetes Care 32, 828–833 (2009).
Mohan, V. et al. A1C cut points to define various glucose intolerance groups in Asian Indians. Diabetes Care 33, 515–519 (2010).
Bennett, C. M., Guo, M. & Dharmage, S. C. HbA1c as a screening tool for detection of type 2 diabetes: a systematic review. Diabet. Med. 24, 333–343 (2007).
Lauritzen, T., Sandbaek, A., Skriver, M. V. & Borch-Johnsen, K. HbA1c and cardiovascular risk score identify people who may benefit from preventive interventions: a 7 year follow-up of a high-risk screening programme for diabetes in primary care (ADDITION), Denmark. Diabetologia 54, 1318–1326 (2011).
Mann, D. M. et al. Impact of A1C screening criterion on the diagnosis of pre-diabetes among U.S. adults. Diabetes Care 33, 2190–2195 (2010).
Gerstein, H. C. et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res. Clin. Pract. 78, 305–312 (2007).
International Diabetes Federation. IDF Diabetes Atlas (4th Edn). Diabetes estimates Excel tables [online]. (2009).
Mozaffarian, D. et al. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch. Intern. Med. 169, 798–807 (2009).
Gillies, C. L. et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 334, 299 (2007).
Lindström, J. et al. Determinants for the effectiveness of lifestyle intervention in the Finnish Diabetes Prevention Study. Diabetes Care 31, 857–862 (2008).
Buijsse, B., Simmons, R. K., Griffin, S. J. & Schulze, M. B. Risk assessment tools for identifying individuals at risk of developing type 2 diabetes. Epidemiol. Rev. 33, 46–62 (2011).
Chen, L. et al. Maximizing efficiency and cost-effectiveness of type 2 diabetes screening: the AusDiab study. Diabet. Med. 28, 414–423 (2011).
Saaristo, T. et al. Lifestyle intervention for prevention of type 2 diabetes in primary health care: one-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D). Diabetes Care 33, 2146–2151 (2010).
Makrilakis, K., Liatis, S., Grammatikou, S., Perrea, D. & Katsilambros, N. Implementation and effectiveness of the first community lifestyle intervention programme to prevent type 2 diabetes in Greece. The DE-PLAN study. Diabet. Med. 27, 459–465 (2010).
Rose, G. Sick individuals and sick populations. Int. J. Epidemiol. 14, 32–38 (1985).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspect of article preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Chen, L., Magliano, D. & Zimmet, P. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol 8, 228–236 (2012). https://doi.org/10.1038/nrendo.2011.183
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2011.183
This article is cited by
-
Type 1 diabetes, its complications, and non-ischemic cardiomyopathy: a mendelian randomization study of European ancestry
Cardiovascular Diabetology (2024)
-
Novel synthesis of AuPt bimetallic nanocubes combined with graphene quantum dots for non-enzymatic sensor of glucose determination by electrochemical method
Chemical Papers (2024)
-
Telecoaching as a new training method for elderly people: a systematic review
Aging Clinical and Experimental Research (2024)
-
Glycemic homeostasis of ultrasound-assisted Murraya koenigii (Linn.) Spreng. bark extract in streptozocin-induced diabetes in rats
Journal of Umm Al-Qura University for Applied Sciences (2024)
-
Subcutaneously transplanted xenogeneic protein recruits treg cells and M2 macrophages to induce browning of inguinal white adipose tissue
Endocrine (2024)