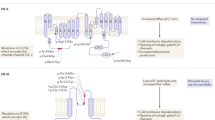
Abstract
In the setting of primary aldosteronism, elevated aldosterone levels are associated with increased blood pressure. Aldosterone concentrations within the normal range, however, can also alter blood pressure. Furthermore, the aldosterone-to-renin ratio, an indicator of aldosterone excess, is associated with hypertension, even in patients without excessive absolute aldosterone levels. In this Review we assess the data on the role of aldosterone in the development and maintenance of hypertension. We provide an overview of the complex crosstalk between genetic and environmental factors, and about aldosterone-mediated arterial hypertension and target organ damage. The discussion is organized according to major targets of aldosterone action: the collecting duct in the kidney, the vasculature and the central nervous system. The antihypertensive efficacy of mineralocorticoid-receptor blockers, even in patients with aldosterone values in the normal range, supports the evidence that aldosterone plays a part in blood pressure elevation in the absence of primary aldosteronism.
Key Points
-
Clinical and experimental evidence shows that aldosterone makes a major contribution beyond primary aldosteronism to the pathogenesis of arterial hypertension
-
Aldosterone excess contributes to endothelial dysfunction, inflammation and vascular remodeling, which result in the development and maintenance of arterial hypertension, via genomic and nongenomic pathways
-
Aldosterone-mediated effects on epithelial and nonepithelial tissues depend on mineralocorticoid sensitivity, which is modulated by salt and angiotensin II
-
Renal Na+ and fluid retention, endothelial dysfunction, increased peripheral resistance and central sympathetic drive are major pathogenic pathways of aldosterone-induced arterial hypertension
-
Mineralocorticoid-receptor blockers are useful drugs to treat aldosterone-mediated arterial hypertension, especially when other antihypertensive drugs insufficiently control blood pressure
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
WHO. World Health Report 2002: reducing risks, promoting healthy life (WHO, Geneva, 2002).
Calhoun, D. A. et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 117, e510–e526 (2008).
Bramlage, P. et al. Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. Am. J. Hypertens. 17, 904–910 (2004).
Perkovic, V., Huxley, R., Wu, Y., Prabhakaran, D. & MacMahon, S. The burden of blood pressure-related disease: a neglected priority for global health. Hypertension 50, 991–997 (2007).
Sarafidis, P. A. & Bakris, G. L. Resistant hypertension: an overview of evaluation and treatment. J. Am. Coll. Cardiol. 52, 1749–1757 (2008).
Vasan, R. S. et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N. Engl. J. Med. 351, 33–41 (2004).
Ingelsson, E. et al. Multimarker approach to evaluate the incidence of the metabolic syndrome and longitudinal changes in metabolic risk factors: the Framingham Offspring Study. Circulation 116, 984–992 (2007).
Kappert, K. & Unger, T. Role of the renin-angiotensin system in hypertension. Hot Topics in Hypertension [online], (2008).
Pratt, J. H. Central role for ENaC in development of hypertension. J. Am. Soc. Nephrol. 16, 3154–3159 (2005).
Connell, J. M., MacKenzie, S. M., Freel, E. M., Fraser, R. & Davies, E. A lifetime of aldosterone excess: long-term consequences of altered regulation of aldosterone production for cardiovascular function. Endocr. Rev. 29, 133–154 (2008).
Ehrhart-Bornstein, M. et al. Human adipocytes secrete mineralocorticoid-releasing factors. Proc. Natl Acad. Sci. USA 100, 14211–14216 (2003).
Oelkers, W. et al. Sensitization of the adrenal cortex to angiotensin II in sodium-deplete man. Circ. Res. 40, 69–77 (1974).
Schlaich, M. P., Schobel, H. P., Hilgers, K. & Schmieder, R. E. Impact of aldosterone on left ventricular structure and function in young normotensive and mildly hypertensive subjects. Am. J. Cardiol. 85, 1199–1206 (2000).
Intersalt Cooperative Research Group. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ 297, 319–328 (1988).
Stamler, J. et al. INTERMAP: background, aims, design, methods, and descriptive statistics (nondietary). J. Hum. Hypertens. 17, 591–608 (2003).
Mohan, S. & Campbell, N. R. Salt and high blood pressure. Clin. Sci. (Lond.) 117, 1–11 (2009).
Meland, E. & Aamland, A. Salt restriction among hypertensive patients: modest blood pressure effect and no adverse effects. Scand. J. Prim. Health Care 27, 97–103 (2009).
Arriza, J. L. et al. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 237, 268–275 (1987).
Funder, J. W. The nongenomic actions of aldosterone. Endocr. Rev. 26, 313–321 (2005).
Grossmann, C. & Gekle, M. New aspects of rapid aldosterone signaling. Mol. Cell. Endocrinol. 308, 53–62 (2009).
Lemarie, C. A., Paradis, P. & Schiffrin, E. L. New insights on signaling cascades induced by cross-talk between angiotensin II and aldosterone. J. Mol. Med. 86, 673–678 (2008).
Jaffe, I. Z. & Mendelsohn, M. E. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ. Res. 96, 643–650 (2005).
Montezano, A. C. & Touyz, R. M. Networking between systemic angiotensin II and cardiac mineralocorticoid receptors. Hypertension 52, 1016–1018 (2008).
Montezano, A. C. et al. Aldosterone and angiotensin II synergistically stimulate migration in vascular smooth muscle cells through c-Src-regulated redox-sensitive RhoA pathways. Arterioscler. Thromb. Vasc. Biol. 28, 1511–1518 (2008).
Harada, E. et al. Aldosterone induces angiotensin-converting-enzyme gene expression in cultured neonatal rat cardiocytes. Circulation 104, 137–139 (2001).
Chai, W. et al. Nongenomic effects of aldosterone in the human heart: interaction with angiotensin II. Hypertension 46, 701–706 (2005).
Xiao, F., Puddefoot, J. R., Barker, S. & Vinson, G. P. Mechanism for aldosterone potentiation of angiotensin II-stimulated rat arterial smooth muscle cell proliferation. Hypertension 44, 340–345 (2004).
Grossmann, C. et al. Aldosterone-induced EGFR expression: interaction between the human mineralocorticoid receptor and the human EGFR promoter. Am. J. Physiol. Endocrinol. Metab. 292, E1790–E1800 (2007).
Ying, W. Z. & Sanders, P. W. Enhanced expression of EGF receptor in a model of salt-sensitive hypertension. Am. J. Physiol. Renal Physiol. 289, F314–F321 (2005).
Min, L. J. et al. Aldosterone and angiotensin II synergistically induce mitogenic response in vascular smooth muscle cells. Circ. Res. 97, 434–442 (2005).
Nagase, M., Matsui, H., Shibata, S., Gotoda, T. & Fujita, T. Salt-induced nephropathy in obese spontaneously hypertensive rats via paradoxical activation of the mineralocorticoid receptor: role of oxidative stress. Hypertension 50, 877–883 (2007).
Kitiyakara, C. et al. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J. Am. Soc. Nephrol. 14, 2775–2782 (2003).
Funder, J. W. Reconsidering the roles of the mineralocorticoid receptor. Hypertension 53, 286–290 (2009).
Mizuno, Y. et al. Aldosterone production is activated in failing ventricle in humans. Circulation 103, 72–77 (2001).
Nishikawa, T. et al. Human renal mesangial cells produce aldosterone in response to low-density lipoprotein (LDL). J. Steroid Biochem. Mol. Biol. 96, 309–316 (2005).
He, F. J. & MacGregor, G. A. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J. Hum. Hypertens. 23, 363–384 (2009).
Nowaczynski, W., Oliver, W. J. & Neel, J. V. Serum aldosterone and protein-binding variables in Yanomama Indians: a no-salt culture as compared to partially acculturated Guaymi Indians. Clin. Physiol. Biochem. 3, 289–306 (1985).
Guyton, A. C. Kidneys and fluids in pressure regulation. Small volume but large pressure changes. Hypertension 19 (Suppl.), I2–I8 (1992).
Machnik, A. et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat. Med. 15, 545–552 (2009).
Schiffrin, E. L. Effects of aldosterone on the vasculature. Hypertension 47, 312–318 (2006).
Cooper, S. A. et al. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am. J. Physiol. Heart Circ. Physiol. 293, H2009–H2023 (2007).
Skott, O. et al. Rapid actions of aldosterone in vascular health and disease—friend or foe? Pharmacol. Ther. 111, 495–507 (2006).
Gekle, M. & Grossmann, C. Actions of aldosterone in the cardiovascular system: the good, the bad, and the ugly? Pflugers Arch. 458, 231–246 (2009).
Arima, S. et al. Nongenomic vascular action of aldosterone in the glomerular microcirculation. J. Am. Soc. Nephrol. 14, 2255–2263 (2003).
Nishizaka, M. K., Zaman, M. A., Green, S. A., Renfroe, K. Y. & Calhoun, D. A. Impaired endothelium-dependent flow-mediated vasodilation in hypertensive subjects with hyperaldosteronism. Circulation 109, 2857–2861 (2004).
Ahmad, N., Romero, D. G., Gomez-Sanchez, E. P. & Gomez-Sanchez, C. E. Do human vascular endothelial cells produce aldosterone? Endocrinology 145, 3626–3629 (2004).
Oberleithner, H. et al. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc. Natl Acad. Sci. USA 104, 16281–16286 (2007).
Oberleithner, H., Riethmuller, C., Ludwig, T., Hausberg, M. & Schillers, H. Aldosterone remodels human endothelium. Acta Physiol. (Oxf.) 187, 305–312 (2006).
Brown, N. J. Aldosterone and vascular inflammation. Hypertension 51, 161–167 (2008).
Ma, L. J. & Fogo, A. B. PAI-1 and kidney fibrosis. Front. Biosci. 14, 2028–2041 (2009).
Rocha, R. et al. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am. J. Physiol. Heart Circ. Physiol. 283, H1802–H1810 (2002).
Blasi, E. R. et al. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 63, 1791–1800 (2003).
Kotchen, T. A., Kotchen, J. M., Grim, C. E., Krishnaswami, S. & Kidambi, S. Aldosterone and alterations of hypertension-related vascular function in African Americans. Am. J. Hypertens. 22, 319–324 (2009).
Jaffe, I. Z., Tintut, Y., Newfell, B. G., Demer, L. L. & Mendelsohn, M. E. Mineralocorticoid receptor activation promotes vascular cell calcification. Arterioscler. Thromb. Vasc. Biol. 27, 799–805 (2007).
Gomez-Sanchez, C. E. et al. Aldosterone biosynthesis in the rat brain. Endocrinology 138, 3369–3373 (1997).
Geerling, J. C. & Loewy, A. D. Aldosterone in the brain. Am. J. Physiol. Renal Physiol. 297, F559–F576 (2009).
Huang, B. S., Wang, H. & Leenen, F. H. Chronic central infusion of aldosterone leads to sympathetic hyperreactivity and hypertension in Dahl S but not Dahl R rats. Am. J. Physiol. Heart Circ. Physiol. 288, H517–H524 (2005).
Minoura, Y., Onimaru, H., Iigaya, K., Homma, I. & Kobayashi, Y. Eectrophysiologic responses of sympathetic preganglionic neurons to angiotensin II and aldosterone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R699–R706 (2009).
Huang, B. S. et al. Activation of brain renin-angiotensin-aldosterone system by central sodium in Wistar rats. Am. J. Physiol. Heart Circ. Physiol. 291, H1109–H1117 (2006).
Huang, B. S., White, R. A., Jeng, A. Y. & Leenen, F. H. Role of central nervous system aldosterone synthase and mineralocorticoid receptors in salt-induced hypertension in Dahl salt-sensitive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R994–R1000 (2009).
Ye, P. et al. Regulation of aldosterone synthase gene expression in the rat adrenal gland and central nervous system by sodium and angiotensin II. Endocrinology 144, 3321–3328 (2003).
Sakai, R. R., McEwen, B. S., Fluharty, S. J. & Ma, L. Y. The amygdala: site of genomic and nongenomic arousal of aldosterone-induced sodium intake. Kidney Int. 57, 1337–1345 (2000).
Ganong, W. F. Circumventricular organs: definition and role in the regulation of endocrine and autonomic function. Clin. Exp. Pharmacol. Physiol. 27, 422–427 (2000).
Francis, J. et al. Central mineralocorticoid receptor blockade improves volume regulation and reduces sympathetic drive in heart failure. Am. J. Physiol. Heart Circ. Physiol. 281, H2241–H2251 (2001).
Geerling, J. C., Engeland, W. C., Kawata, M. & Loewy, A. D. Aldosterone target neurons in the nucleus tractus solitarius drive sodium appetite. J. Neurosci. 26, 411–417 (2006).
Reynolds, R. M. et al. Programming of hypertension: associations of plasma aldosterone in adult men and women with birthweight, cortisol, and blood pressure. Hypertension 53, 932–936 (2009).
Isaji, M. et al. Correlation between left ventricular mass and urinary sodium excretion in specific genotypes of CYP11B2. J. Hypertens. 23, 1149–1157 (2005).
Lim, P. O. et al. Variation at the aldosterone synthase (CYP11B2) locus contributes to hypertension in subjects with a raised aldosterone-to-renin ratio. J. Clin. Endocrinol. Metab. 87, 4398–4402 (2002).
Tsujita, Y. et al. Lack of association between genetic polymorphism of CYP11B2 and hypertension in Japanese: the Suita Study. Hypertens. Res. 24, 105–109 (2001).
Davies, E. et al. Aldosterone excretion rate and blood pressure in essential hypertension are related to polymorphic differences in the aldosterone synthase gene CYP11B2. Hypertension 33, 703–707 (1999).
Castellano, M. et al. Genetic polymorphism of the renin-angiotensin-aldosterone system and arterial hypertension in the Italian population: the GENIPER Project. J. Hypertens. 21, 1853–1860 (2003).
Nejatizadeh, A. et al. CYP11B2 gene haplotypes independently and in concurrence with aldosterone and aldosterone to renin ratio increase the risk of hypertension. Clin. Biochem. doi:10.1016/j.clinbiochem.2009.09.015.
Sookoian, S., Gianotti, T. F., Gonzalez, C. D. & Pirola, C. J. Association of the C-344T aldosterone synthase gene variant with essential hypertension: a meta-analysis. J. Hypertens. 25, 5–13 (2007).
Makhanova, N., Hagaman, J., Kim, H. S. & Smithies, O. Salt-sensitive blood pressure in mice with increased expression of aldosterone synthase. Hypertension 51, 134–140 (2008).
Schlaich, M. P. et al. Altered aldosterone response to salt intake and angiotensin II infusion in young normotensive men with parental history of arterial hypertension. J. Hypertens. 20, 117–124 (2002).
Kathiresan, S. et al. Clinical and genetic correlates of serum aldosterone in the community: the Framingham Heart Study. Am. J. Hypertens. 18, 657–665 (2005).
Kotchen, T. A. et al. Genetic determinants of hypertension: identification of candidate phenotypes. Hypertension 36, 7–13 (2000).
Inglis, G. C. et al. Familial pattern of corticosteroids and their metabolism in adult human subjects—the Scottish Adult Twin Study. J. Clin. Endocrinol. Metab. 84, 4132–4137 (1999).
Newton-Cheh, C. et al. Clinical and genetic correlates of aldosterone-to-renin ratio and relations to blood pressure in a community sample. Hypertension 49, 846–856 (2007).
Gaddam, K. K. et al. Characterization of resistant hypertension: association between resistant hypertension, aldosterone, and persistent intravascular volume expansion. Arch. Intern. Med. 168, 1159–1164 (2008).
Lin, L. et al. Severe loss-of-function mutations in the adrenocorticotropin receptor (ACTHR, MC2R) can be found in patients diagnosed with salt-losing adrenal hypoplasia. Clin. Endocrinol. (Oxf.) 66, 205–210 (2007).
Luft, F. C. & Weinberger, M. H. Heterogeneous responses to changes in dietary salt intake: the salt-sensitivity paradigm. Am. J. Clin. Nutr. 65 (Suppl.), S612–S617 (1997).
Hannila-Handelberg, T. et al. Common variants of the beta and gamma subunits of the epithelial sodium channel and their relation to plasma renin and aldosterone levels in essential hypertension. BMC Med. Genet. 6, 4 (2005).
Shimkets, R. A. et al. Liddle's syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 79, 407–414 (1994).
von Wowern, F. et al. Genetic variance of SGK-1 is associated with blood pressure, blood pressure change over time and strength of the insulin-diastolic blood pressure relationship. Kidney Int. 68, 2164–2172 (2005).
Kucharz, E. J. Michal Litynski—a forgotten author of the first description on primary hyperaldosteronism [Polish]. Pol. Arch. Med. Wewn. 117, 57–58 (2007).
Conn, J. W. & Louis, L. H. Primary aldosteronism, a new clinical entity. Ann. Intern. Med. 44, 1–15 (1956).
Genest, J. et al. Human arterial hypertension: a state of mild chronic hyperaldosteronism? Science 123, 503–505 (1956).
Russell, R. P. & Masi, A. T. Significant associations of adrenal cortical abnormalities with “essential” hypertension. Am. J. Med. 54, 44–51 (1973).
Mosso, L. et al. Primary aldosteronism and hypertensive disease. Hypertension 42, 161–165 (2003).
Pilz, S. et al. Graz Endocrine Causes of Hypertension (GECOH) study: a diagnostic accuracy study of aldosterone to active renin ratio in screening for primary aldosteronism. BMC Endocr. Disord. 9, 11 (2009).
Calhoun, D. A., Nishizaka, M. K., Zaman, M. A., Thakkar, R. B. & Weissmann, P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension 40, 892–896 (2002).
Calhoun, D. A. Is there an unrecognized epidemic of primary aldosteronism? Pro. Hypertension 50, 447–453 (2007).
Kaplan, N. M. Is there an unrecognized epidemic of primary aldosteronism? Con. Hypertension 50, 454–458 (2007).
Fiquet-Kempf, B., Launay-Mignot, P., Bobrie, G. & Plouin, P. F. Is primary aldosteronism underdiagnosed in clinical practice? Clin. Exp. Pharmacol. Physiol. 28, 1083–1086 (2001).
Sartori, M. et al. Aldosterone and refractory hypertension: a prospective cohort study. Am. J. Hypertens. 19, 373–379 (2006).
Meneton, P. et al. High plasma aldosterone and low renin predict blood pressure increase and hypertension in middle-aged Caucasian populations. J. Hum. Hypertens. 22, 550–558 (2008).
Lieb, W. et al. Multimarker approach to evaluate correlates of vascular stiffness: the Framingham Heart Study. Circulation 119, 37–43 (2009).
Tomaschitz, A. et al. How does the aldosterone renin ratio impact blood pressure levels? A cross-sectional study of 3056 normo- and hypertensive patients referred to coronary angiography. Endocrine Abstracts 16, P9 (2008).
Lim, P. O., Struthers, A. D. & MacDonald, T. M. The neurohormonal natural history of essential hypertension: towards primary or tertiary aldosteronism? J. Hypertens. 20, 11–15 (2002).
Eide, I. K., Torjesen, P. A., Drolsum, A., Babovic, A. & Lilledahl, N. P. Low-renin status in therapy-resistant hypertension: a clue to efficient treatment. J. Hypertens. 22, 2217–2226 (2004).
Mulatero, P. et al. CYP11B2 gene polymorphisms in idiopathic hyperaldosteronism. Hypertension 35, 694–698 (2000).
Pratt, J. H. Low-renin hypertension: more common than we think? Cardiol. Rev. 8, 202–206 (2000).
Pimenta, E. et al. Aldosterone excess and resistance to 24-h blood pressure control. J. Hypertens. 25, 2131–2137 (2007).
Lastra-Lastra, G., Sowers, J. R., Restrepo-Erazo, K., Manrique-Acevedo, C. & Lastra-Gonzalez, G. Role of aldosterone and angiotensin II in insulin resistance: an update. Clin. Endocrinol. (Oxf.) 71, 1–6 (2009).
Hitomi, H. et al. Aldosterone suppresses insulin signaling via the downregulation of insulin receptor substrate-1 in vascular smooth muscle cells. Hypertension 50, 750–755 (2007).
Mosso, L. M. et al. A possible association between primary aldosteronism and a lower beta-cell function. J. Hypertens. 25, 2125–2130 (2007).
Bochud, M. et al. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension 48, 239–245 (2006).
Goodfriend, T. L. & Calhoun, D. A. Resistant hypertension, obesity, sleep apnea, and aldosterone: theory and therapy. Hypertension 43, 518–524 (2004).
Sowers, J. R., Whaley-Connell, A. & Epstein, M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann. Intern. Med. 150, 776–783 (2009).
Tolagen, K. & Karlberg, B. E. Plasma and urinary aldosterone and their interrelations with blood pressure, plasma renin activity and urinary electrolytes in normotensive subjects. Scand. J. Clin. Lab. Invest. 38, 241–247 (1978).
Duprez, D. A. et al. Influence of arterial blood pressure and aldosterone on left ventricular hypertrophy in moderate essential hypertension. Am. J. Cardiol. 71, 17A–20A (1993).
Walker, W. G., Whelton, P. K., Saito, H., Russell, R. P. & Hermann, J. Relation between blood pressure and renin, renin substrate, angiotensin II, aldosterone and urinary sodium and potassium in 574 ambulatory subjects. Hypertension 1, 287–291 (1979).
Grim, C. E. et al. Hyperaldosteronism and hypertension: ethnic differences. Hypertension 45, 766–772 (2005).
Di Zhang, A. et al. Cross-talk between mineralocorticoid and angiotensin II signaling for cardiac remodeling. Hypertension 52, 1060–1067 (2008).
Virdis, A. et al. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension 40, 504–510 (2002).
Lea, W. B. et al. Aldosterone antagonism or synthase inhibition reduces end-organ damage induced by treatment with angiotensin and high salt. Kidney Int. 75, 936–944 (2009).
Fujita, T. Aldosterone in salt-sensitive hypertension and metabolic syndrome. J. Mol. Med. 86, 729–734 (2008).
Douglas, J. G., Hollifield, J. W. & Liddle, G. W. Treatment of low-renin essential hypertension. Comparison of spironolactone and a hydrochlorothiazide-triamterene combination. JAMA 227, 518–521 (1974).
Hood, S. J., Taylor, K. P., Ashby, M. J. & Brown, M. J. The spironolactone, amiloride, losartan, and thiazide (SALT) double-blind crossover trial in patients with low-renin hypertension and elevated aldosterone-renin ratio. Circulation 116, 268–275 (2007).
Nishizaka, M. K., Zaman, M. A. & Calhoun, D. A. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am. J. Hypertens. 16, 925–930 (2003).
Lane, D. A., Shah, S. & Beevers, D. G. Low-dose spironolactone in the management of resistant hypertension: a surveillance study. J. Hypertens. 25, 891–894 (2007).
Chapman, N. et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 49, 839–845 (2007).
Gross, E., Rothstein, M., Dombek, S. & Juknis, H. I. Effect of spironolactone on blood pressure and the renin-angiotensin-aldosterone system in oligo-anuric hemodialysis patients. Am. J. Kidney Dis. 46, 94–101 (2005).
Saruta, T. et al. Efficacy and safety of the selective aldosterone blocker eplerenone in Japanese patients with hypertension: a randomized, double-blind, placebo-controlled, dose-ranging study. J. Clin. Hypertens. (Greenwich) 6, 175–185 (2004).
Weinberger, M. H., Roniker, B., Krause, S. L. & Weiss, R. J. Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am. J. Hypertens. 15, 709–716 (2002).
White, W. B. et al. Effects of the selective aldosterone blocker eplerenone versus the calcium antagonist amlodipine in systolic hypertension. Hypertension 41, 1021–1026 (2003).
Pitt, B. et al. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation 108, 1831–1838 (2003).
Weinberger, M. H. et al. Effects of eplerenone versus losartan in patients with low-renin hypertension. Am. Heart J. 150, 426–433 (2005).
Flack, J. M. et al. Efficacy and tolerability of eplerenone and losartan in hypertensive black and white patients. J. Am. Coll. Cardiol. 41, 1148–1155 (2003).
Krum, H. et al. Efficacy of eplerenone added to renin-angiotensin blockade in hypertensive patients. Hypertension 40, 117–123 (2002).
Imanishi, T. et al. Addition of eplerenone to an angiotensin-converting enzyme inhibitor effectively improves nitric oxide bioavailability. Hypertension 51, 734–741 (2008).
Lim, P. O., Jung, R. T. & MacDonald, T. M. Raised aldosterone to renin ratio predicts antihypertensive efficacy of spironolactone: a prospective cohort follow-up study. Br. J. Clin. Pharmacol. 48, 756–760 (1999).
Prisant, L. M. et al. Can renin status predict the antihypertensive efficacy of eplerenone add-on therapy? J. Clin. Pharmacol. 43, 1203–1210 (2003).
Mahmud, A., Mahgoub, M., Hall, M. & Feely, J. Does aldosterone-to-renin ratio predict the antihypertensive effect of the aldosterone antagonist spironolactone? Am. J. Hypertens. 18, 1631–1635 (2005).
Weinberger, M. H. The use of selective aldosterone antagonists. Curr. Hypertens. Rep. 6, 342–345 (2004).
Burgess, E. Eplerenone in hypertension. Expert Opin. Pharmacother. 5, 2573–2581 (2004).
Jansen, P. M., Danser, A. H., Imholz, B. P. & van den Meiracker, A. H. Aldosterone-receptor antagonism in hypertension. J. Hypertens. 27, 680–691 (2009).
Juurlink, D. N. et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N. Engl. J. Med. 351, 543–551 (2004).
Saha, C. et al. Improvement in blood pressure with inhibition of the epithelial sodium channel in blacks with hypertension. Hypertension 46, 481–487 (2005).
Pratt, J. H., Eckert, G. J., Newman, S. & Ambrosius, W. T. Blood pressure responses to small doses of amiloride and spironolactone in normotensive subjects. Hypertension 38, 1124–1129 (2001).
Ullian, M. E. & Fine, J. J. Mechanisms of enhanced angiotensin II-stimulated signal transduction in vascular smooth muscle by aldosterone. J. Cell. Physiol. 161, 201–208 (1994).
Oberleithner, H. et al. Human endothelium: target for aldosterone. Hypertension 43, 952–956 (2004).
Kidambi, S. et al. Association of adrenal steroids with hypertension and the metabolic syndrome in blacks. Hypertension 49, 704–711 (2007).
Schunkert, H., Hense, H. W., Andus, T., Riegger, G. A. & Straub, R. H. Relation between dehydroepiandrosterone sulfate and blood pressure levels in a population-based sample. Am. J. Hypertens. 12, 1140–1143 (1999).
El-Gharbawy, A. H. et al. Arterial pressure, left ventricular mass, and aldosterone in essential hypertension. Hypertension 37, 845–850 (2001).
Ljungman, S., Aurell, M., Hartford, M., Wikstrand, J. & Berglund, G. Blood pressure in relation to the renin-angiotensin-aldosterone system. Acta Med. Scand. 211, 351–360 (1982).
Lamarre-Cliche, M. et al. Effects of circadian rhythms, posture, and medication on renin-aldosterone interrelations in essential hypertensives. Am. J. Hypertens. 18, 56–64 (2005).
Acknowledgements
A. Tomaschitz and S. Pilz have contributed equally in drafting the initial version of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Tomaschitz, A., Pilz, S., Ritz, E. et al. Aldosterone and arterial hypertension. Nat Rev Endocrinol 6, 83–93 (2010). https://doi.org/10.1038/nrendo.2009.263
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2009.263