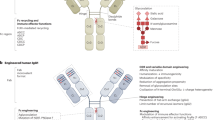
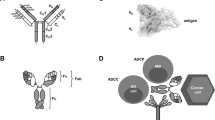
Abstract
Monoclonal antibodies are now established as a key therapeutic modality for a range of diseases. Owing to the ability of these agents to selectively target tumour cells, cancer has been a major focus of development programmes for monoclonal antibodies so far. Here, we overview trends in the clinical development and regulatory approval of monoclonal antibodies for cancer since 1980, with the aim of informing future research and development for this class of therapeutics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Reichert, J. M., Rosensweig, C. J., Faden, L. B. & Dewitz, M. C. Monoclonal antibody successes in the clinic. Nature Biotech. 23, 1073–1078 (2005).
Lin, M. Z., Teitell, M. A. & Schiller, G. J. The evolution of antibodies into versatile tumor-targeting agents. Clin. Cancer Res. 11, 129–38 (2005).
Carter, P. Improving the efficacy of antibody-based cancer therapies. Nature Rev. Cancer 1, 118–129 (2001).
Carter, P., Smith, L. & Ryan, M. Identification and validation of cell surface antigens for antibody targeting in oncology. Endocr. Relat. Cancer 11, 659–687 (2004).
Punt, C. J. et al. Edrecolomab alone or in combination with fluorouracil and folinic acid in the adjuvant treatment of stage III colon cancer: a randomised study. Lancet 360, 671–677 (2002).
Kohler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975).
Ober, R. J., Radu, C. G., Ghetie, V. & Ward, E. S. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int. Immunol. 13, 1551–1559 (2001).
Shawler, D. L, Bartholomew, R. M., Smith, L. M. & Dillman, R. O. Human immune response to multiple injections of murine monoclonal IgG. J. Immunol. 135, 1530–1535 (1985).
Kuus-Reichel, K. et al. Will immunogenicity limit the use, efficacy, and future development of therapeutic monoclonal antibodies? Clin. Diagn. Lab. Immunol. 1, 365–372 (1994).
Kipps, T. J., Parham, P., Punt, J. & Herzenberg, L. A. Importance of immunoglobulin isotype in human antibody-dependent, cell-mediated cytotoxicity directed by murine monoclonal antibodies. J Exp. Med. 161, 1–17 (1985).
Presta, L. G. Engineering of therapeutic antibodies to minimize immunogenicity and optimize function. Adv. Drug Del. Rev. 58, 640–656 (2006).
Morrison, S. L., Johnson, M. J., Herzenberg, L. A. & Oi, V. T. Chimeric human antibody molecules: mouse antigen-binding domains with human constant domains. Proc. Natl Acad. Sci. USA 81, 6851–6855 (1984).
Jones, P. T., Dear, P. H., Foote, J., Neuberger, M. S. & Winter, G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 321, 522–525 (1986).
Reichmann, L., Clark, M., Waldmann, H. & Winter, G. Reshaping human antibodies for therapy. Nature 332, 323–327 (1988).
Lonberg, N. Human antibodies from transgenic animals. Nature Biotech. 23, 1117–1125 (2005).
McCafferty, J., Griffiths, A. D., Winter, G. & Chiswell, D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature 348, 552–554 (1990).
Payne, G. Progress in immunoconjugate cancer therapeutics. Cancer Cell 3, 207–212 (2003).
Schrama, D., Reisfeld, R. A. & Becker, J. C. Antibody targeted drugs as cancer therapeutics. Nature Rev. Drug Discov. 5, 147–159 (2006).
Milenic, D. E., Brady, E. D. & Brechbiel, M. W. Antibody-targeted radiation cancer therapy. Nature Rev. Drug Discov. 3, 488–499 (2004).
Sharkey, R. M. & Goldenberg, D. M. Perspectives on cancer therapy with radiolabeled monoclonal antibodies. J. Nucl. Med. 46, 115S–127S (2005).
Goldenberg, D. M. Targeted therapy of cancer with radiolabeled antibodies. J. Nucl. Med. 43, 693–713 (2002).
Frankel, A. E., Kreitman, R. J. & Sausville, E. A. Targeted toxins. Clin. Cancer Res. 6, 326–334 (2000).
Morell, A., Terry, W. D. & Waldmann, T. A. Metabolic properties of IgG subclasses in man J. Clin. Invest. 49, 673–680 (1970).
Adams, G. P. & Weiner, L. M. Monoclonal antibody therapy of cancer. Nature Biotech. 23, 1147–1157 (2005).
Stern, M. & Herrmann, R. Overview of monoclonal antibodies in cancer therapy: present and promise. Crit. Rev. Oncol. Hematol. 54, 11–29 (2005).
Trikha, M., Yan, L. & Nakada M. T. Monoclonal antibodies as therapeutics in oncology. Curr. Opin. Biotechnol. 13, 609–614 (2002).
Gelderman, K. A., Tomlinson, S., Ross, G. D. & Gorter, A. Complement function in mAb-mediated cancer immunotherapy. Trends Immunol. 25, 158–164 (2004).
Bruggeman, M. et al. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J. Exp. Med. 166, 1351–1361 (1987).
Nimmerjahn, F. & Ravetch, J. V. Divergent immunoglobulin G subclass activity through selective Fc receptor binding. Science 310, 1510–1512 (2005).
Gennari, R. et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin. Cancer Res. 10, 5650–5655 (2004).
Janas, E. et al. Rituxan (anti-CD20 antibody)-induced translocation of CD20 into lipid rafts is crucial for calcium influx and apoptosis. Clin. Exp. Immunol. 139, 439–446 (2005).
Scott, A. M. et al. Construction, production, and characterization of humanized anti-Lewis Y monoclonal antibody 3S193 for targeted immunotherapy of solid tumors. Cancer Res. 60, 3254–3261 (2000).
Harris, M. Monoclonal antibodies as therapeutic agents for cancer. Lancet Oncol. 5, 292–302 (2004).
Spicer, J. Technology evaluation: nimotuzumab, the Center of Molecular Immunology/YM BioSciences/Oncoscience. Curr. Opin. Mol. Ther. 7, 182–191 (2005).
Nahta, R. & Esteva, F. J. Herceptin: mechanisms of action and resistance. Cancer Lett. 232, 123–138 (2006).
Arnould, L. et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br. J. Cancer 94, 259–267 (2006).
Salgaller, M. L. Technology evaluation: bevacizumab, Genentech/Roche. Curr. Opin. Mol. Ther. 5, 657–667 (2003).
Pukac, L. et al. HGS-ETR1, a fully human TRAIL-receptor 1 monoclonal antibody, induces cell death in multiple tumour types in vitro and in vivo. Br. J. Cancer 92, 1430–1441 (2005).
Melero, I. et al. Immunostimulatory monoclonal antibodies for cancer therapy. Nature Rev. Cancer 7, 95–106 (2007).
Leach, D.R., Krummel, H.F. & Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (2006).
Zeidler, R. et al. Simultaneous activation of T cells and accessory cells by a new class of intact bispecific antibody results in efficient tumor cell killing. J. Immunol. 163, 1246–1252 (1999).
Baker, M. Upping the ante on antibodies. Nature Biotech. 23, 1065–1072 (2005).
Hoogenboom, H. R. Selecting and screening recombinant antibody libraries. Nature Biotech. 23, 1105–1116 (2005).
Carter, P. Potent antibody therapeutics by design. Nature Rev. Immunol. 6, 343–357 (2006).
Dancey, J. E. & Chen, H. X. Strategies for optimizing combinations of molecularly targeted anticancer agents. Nature Rev. Drug Discov. 5, 649–659 (2006).
Jimeno, A. & Hidalgo, M. Multitargeted therapy: can promiscuity be praised in an era of political correctness? Crit. Rev. Oncol. Hematol. 59, 150–158 (2006).
Acknowledgements
The authors thank C. Rosensweig for assistance with data collection, D. Glover for comments and suggestions throughout the project, P. Round, C. Smith, as well as colleagues at the Tufts Center for the Study of Drug Development and Cambridge Antibody Technology for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Viia Valge-Archer is employed by Cambridge Antibody Technology.
Related links
Glossary
- Antibodies
-
Complex protein-based molecules produced by B lymphocytes that bind to and help to eliminate foreign and infectious agents in the body. Antibodies are Yshaped, having two sets of branches attached to a single stem. The arms of the Y (Fab) are the socalled variable regions, the tips of the arms contain antigen-binding regions, and the stem (Fc) is a constant region. The constant regions trigger effector functions (phagocytosis, cytolysis or initiation of complement cascade followed by cell lysis) by linking the complex to other cells of the immune system.
- Chimeric monoclonal antibody
-
A monoclonal antibody that is constructed from variable regions that are derived from murine source and constant regions derived from human source.
- Human monoclonal antibody
-
A monoclonal antibody that is derived entirely from a human source, currently transgenic mice or phage display. Human mAbs can also be produced from human hybridomas or human Blymphocyte cell lines immortalized by EpsteinBarr virus. However, these cell lines are unstable and produce only small amounts of mAbs.
- Humanized monoclonal antibody
-
A monoclonal antibody constructed with only antigen-binding regions (also called complementarity determining regions (CDRs)) that are derived from a mouse. The remainder of the variable regions and constant regions are derived from a human source.
- Immunoconjugates
-
A hybrid molecule formed by covalently or chemically joining a therapeutic molecule to an antibody, or antibody fragment, with the aim of targeting the therapeutic to a specific antigen.
- Isotypes
-
Antibody types defined according to their classes and subclasses of heavy and light chain constant domains, which are encoded by distinct genetic loci.
- Monoclonal antibodies
-
Originally, mAbs were antibodies produced from a single B lymphocyte. Genetic manipulation now allows genes from multiple sources of B lymphocytes (for example, mouse and human) to be combined. mAbs of a defined peptide sequence have identical antigen-binding regions and bind to the same site (the epitope) of an antigen.
- Murine monoclonal antibody
-
A monoclonal antibody that is derived entirely from mice; specifically mouse hybridomas generated from the fusion of mouse myeloma and mouse Blymphocyte cells.
- Phage display
-
Expression and display of antibody fragments (or other proteins) on the surface of filamentous bacteriophage, which links the antibody DNA sequence with its function, such as binding to target antigen.
- Primatized monoclonal antibody
-
A monoclonal antibody that is constructed from variable regions that are derived from Cynomolgus macaques and constant regions that are derived from a human source. Primatized is a registered US trademark of Biogen IDEC.
- Transgenic mice
-
Mice carrying foreign genetic material in their germline DNA. Mice capable of producing human antibodies contain gene sequences for human antibody heavy and light chains.
Rights and permissions
About this article
Cite this article
Reichert, J., Valge-Archer, V. Development trends for monoclonal antibody cancer therapeutics. Nat Rev Drug Discov 6, 349–356 (2007). https://doi.org/10.1038/nrd2241
Issue Date:
DOI: https://doi.org/10.1038/nrd2241
This article is cited by
-
miR-124-3p and miR-194-5p regulation of the PI3K/AKT pathway via ROR2 in medulloblastoma progression
Cancer Gene Therapy (2024)
-
Monoclonal Antibodies: Purification, Application in Conventional Methods and Cutting Edge Technology
Biomedical Materials & Devices (2024)
-
Antibody-drug conjugates in cancer therapy: innovations, challenges, and future directions
Archives of Pharmacal Research (2024)
-
FAP is critical for ovarian cancer cell survival by sustaining NF-κB activation through recruitment of PRKDC in lipid rafts
Cancer Gene Therapy (2023)
-
Targeted therapy of angiogenesis using anti-VEGFR2 and anti-NRP-1 nanobodies
Cancer Chemotherapy and Pharmacology (2022)