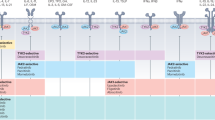
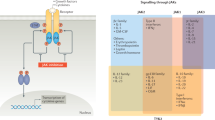
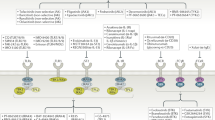
Key Points
-
Despite their success, conventional and biologic disease-modifying antirheumatic drugs are not effective in all patients.
-
First-generation Janus kinase (JAK) inhibitors, or jakinibs, are effective for rheumatoid arthritis and in a number of other autoimmune conditions.
-
Next-generation selective jakinibs are being developed and are effective in rheumatoid arthritis, inflammatory bowel disease and other autoimmune conditions.
-
First-generation and second-generation jakinibs are currently being investigated for a number of new indications.
-
Many of the adverse effects of jakinibs can be linked to the action of the cytokines that are blocked.
-
Topical jakinibs represent an exciting new class of agents that may preserve therapeutic efficacy while eliminating adverse effects that result from systemic JAK inhibition.
Abstract
The discovery of cytokines as key drivers of immune-mediated diseases has spurred efforts to target their associated signalling pathways. Janus kinases (JAKs) are essential signalling mediators downstream of many pro-inflammatory cytokines, and small-molecule inhibitors of JAKs (jakinibs) have gained traction as safe and efficacious options for the treatment of inflammation-driven pathologies such as rheumatoid arthritis, psoriasis and inflammatory bowel disease. Building on the clinical success of first-generation jakinibs, second-generation compounds that claim to be more selective are currently undergoing development and proceeding to clinical trials. However, important questions remain about the advantages and limitations of improved JAK selectivity, optimal routes and dosing regimens and how best to identify patients who will benefit from jakinibs. This Review discusses the biology of jakinibs from a translational perspective, focusing on recent insights from clinical trials, the development of novel agents and the use of jakinibs in a spectrum of immune and inflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
28 December 2017
In the version of this article that was originally published, there was an error in Figure 3 on page 847. In this figure, the structure attributed to the compound BMS-986165 is incorrect and the correct structure for this compound has not yet been disclosed. This error has now been corrected in the online version of the article, and the structure of SAR-20347 has been added instead. We apologize for any inconvenience that this error may have caused.
References
Schwartz, D. M., Bonelli, M., Gadina, M. & O'Shea, J. J. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat. Rev. Rheumatol. 12, 25–36 (2016).
Singh, J. A. et al. Biologics or tofacitinib for rheumatoid arthritis in incomplete responders to methotrexate or other traditional disease-modifying anti-rheumatic drugs: a systematic review and network meta-analysis. Cochrane Database Syst. Rev. 13, CD012183 (2016).
Calabrese, L. H. & Rose-John, S. IL-6 biology: implications for clinical targeting in rheumatic disease. Nat. Rev. Rheumatol. 10, 720–727 (2014).
Pepys, M. B. & Hirschfield, G. M. C-Reactive protein: a critical update. J. Clin. Invest. 111, 1805–1812 (2003).
Feagan, B. G. et al. Ustekinumab as induction and maintenance therapy for Crohn's disease. N. Engl. J. Med. 375, 1946–1960 (2016).
Ortega, H. G. et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 371, 1198–1207 (2014).
Hanania, N. A. et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir. Med. 4, 781–796 (2016).
Wenzel, S. et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 388, 31–44 (2016).
O'Shea, J. J., Holland, S. M. & Staudt, L. M. JAKs and STATs in immunity, immunodeficiency, and cancer. N. Engl. J. Med. 368, 161–170 (2013).
O'Shea, J. J. et al. The JAK–STAT pathway: impact on human disease and therapeutic intervention. Annu. Rev. Med. 66, 311–328 (2015).
Ross, S. H. et al. Phosphoproteomic analyses of interleukin 2 signaling reveal integrated JAK kinase-dependent and -independent networks in CD8+ T cells. Immunity 45, 685–700 (2016).
Macchi, P. et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature 377, 65–68 (1995).
Russell, S. M. et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science 270, 797–800 (1995).
Druker, B. J. et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 344, 1031–1037 (2001).
Rask-Andersen, M., Zhang, J., Fabbro, D. & Schioth, H. B. Advances in kinase targeting: current clinical use and clinical trials. Trends Pharmacol. Sci. 35, 604–620 (2014).
Herrera, A. F. & Jacobsen, E. D. Ibrutinib for the treatment of mantle cell lymphoma. Clin. Cancer Res. 20, 5365–5371 (2014).
Lippert, E. et al. The JAK2-V617F mutation is frequently present at diagnosis in patients with essential thrombocythemia and polycythemia vera. Blood 108, 1865–1867 (2006).
Verstovsek, S. et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med. 366, 799–807 (2012).
Harrison, C. et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N. Engl. J. Med. 366, 787–798 (2012).
Waldmann, T. A. & Chen, J. Disorders of the JAK/STAT pathway in T cell lymphoma pathogenesis: implications for immunotherapy. Annu. Rev. Immunol. 35, 533–550 (2017).
Kudlacz, E. et al. The novel JAK-3 inhibitor CP-690550 is a potent immunosuppressive agent in various murine models. Am. J. Transplant. 4, 51–57 (2004).
Ghoreschi, K. et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J. Immunol. 186, 4234–4243 (2011).
Milici, A. J., Kudlacz, E. M., Audoly, L., Zwillich, S. & Changelian, P. Cartilage preservation by inhibition of Janus kinase 3 in two rodent models of rheumatoid arthritis. Arthritis Res. Ther. 10, R14 (2008).
Lee, E. B. et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N. Engl. J. Med. 370, 2377–2386 (2014). This study establishes the superiority of tofacitinib to methotrexate in the treatment of rheumatoid arthritis.
Burmester, G. R. et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 381, 451–460 (2013). This study establishes the efficacy of tofacitinib in patients who had failed to respond to two or more biological agents.
van Vollenhoven, R. F. et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N. Engl. J. Med. 367, 508–519 (2012). This study finds tofacitinib to be non-inferior to adalimumab as combination treatment with methotrexate in patients with continued disease activity on methotrexate. It establishes the equivalence of jakinibs to standard-of-care treatments.
Lee, E. B. et al. Radiographic, clinical and functional comparison of tofacitinib monotherapy versus methotrexate in methotrexate-nai¨ve patients with rheumatoid arthritis [abstract]. Arthritis Rheumatol. 64 (Suppl. 10), 2486 (2012).
Kremer, J. M. et al. Tofacitinib (cp-690,550), an oral JAK inhibitor, in combination with traditional DMARDs: phase 3 study in patients with active rheumatoid arthritis with inadequate response to DMARDs [abstract]. Ann. Rheum. Dis. 70 (Suppl. 3), 170 (2011).
Fleischmann, R. M. et al. Efficacy of tofacitinib monotherapy in methotrexate-naive patients with early or established rheumatoid arthritis. RMD Open 2, e000262 (2016).
Fleischmann, R. et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N. Engl. J. Med. 367, 495–507 (2012). This phase III trial of tofacitinib in rheumatoid arthritis found tofacitinib to be effective as monotherapy.
He, Y. et al. Efficacy and safety of tofacitinib in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. BMC Musculoskelet. Disord. 14, 298 (2013).
Kremer, J. et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann. Intern. Med. 159, 253–261 (2013).
van der Heijde, D. et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 65, 559–570 (2013).
Conaghan, P. G. et al. Comparing the effects of tofacitinib, methotrexate and the combination, on bone marrow oedema, synovitis and bone erosion in methotrexate-naive, early active rheumatoid arthritis: results of an exploratory randomised MRI study incorporating semiquantitative and quantitative techniques. Ann. Rheum. Dis. 75, 1024–1033 (2016).
Strand, V. et al. Tofacitinib with methotrexate in third-line treatment of patients with active rheumatoid arthritis: patient-reported outcomes from a phase III trial. Arthritis Care Res. 67, 475–483 (2015).
Charles-Schoeman, C. et al. Efficacy and safety of tofacitinib following inadequate response to conventional synthetic or biological disease-modifying antirheumatic drugs. Ann. Rheum. Dis. 75, 1293–1301 (2016).
Landewé, R. B. et al. Is radiographic progression in modern rheumatoid arthritis trials still a robust outcome? Experience from tofacitinib clinical trials. Arthritis Res. Ther. 18, 212 (2016).
Traynor, K. FDA approves tofacitinib for rheumatoid arthritis. Am. J. Health Syst. Pharm. 69, 2120 (2012).
Yamanaka, H. et al. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: an open-label, long-term extension study. Arthritis Res. Ther. 18, 34 (2016).
Wollenhaupt, J. et al. Safety and efficacy of tofacitinib, an oral janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J. Rheumatol 41, 837–852 (2014).
Fleischmann, R. et al. Efficacy and safety of tofacitinib in patients with active rheumatoid arthritis: review of key phase 2 studies. Int. J. Rheum. Dis. 19, 1216–1225 (2016).
Smolen, J. S. et al. Remission rates with tofacitinib treatment in rheumatoid arthritis: a comparison of various remission criteria. Arthritis Rheumatol. 69, 728–734 (2017).
Wang, J., Devenport, J., Low, J. M., Yu, D. & Hitraya, E. Relationship between baseline and early changes in C-reactive protein and interleukin-6 levels and clinical response to tocilizumab in rheumatoid arthritis. Arthritis Care Res. 68, 882–885 (2016).
Strand, V. et al. Tofacitinib versus methotrexate in rheumatoid arthritis: patient-reported outcomes from the randomised phase III ORAL Start trial. RMD Open 2, e000308 (2016).
Strand, V. et al. Effects of tofacitinib monotherapy on patient-reported outcomes in a randomized phase 3 study of patients with active rheumatoid arthritis and inadequate responses to DMARDs. Arthritis Res. Ther. 17, 307 (2015).
Lamba, M. et al. Extended-release once-daily formulation of tofacitinib: evaluation of pharmacokinetics compared with immediate-release tofacitinib and impact of food. J. Clin. Pharmacol. 56, 1362–1371 (2016).
Shi, J. G. et al. The pharmacokinetics, pharmacodynamics, and safety of baricitinib, an oral JAK 1/2 inhibitor, in healthy volunteers. J. Clin. Pharmacol. 54, 1354–1361 (2014).
Dougados, M. et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann. Rheum. Dis. 76, 88–95 (2017).
Fleischmann, R. et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol. 69, 506–517 (2017).
Genovese, M. C. et al. Baricitinib in patients with refractory rheumatoid arthritis. N. Engl. J. Med. 374, 1243–1252 (2016). This phase III trial of baricitinib in rheumatoid arthritis found it to be effective in patients who had not responded to biologic DMARDs.
Taylor, P. C. et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N. Engl. J. Med. 376, 652–662 (2017). This study compares baricitinib to adalimumab or placebo in patients with rheumatoid arthritis who do not respond to treatment with methotrexate. It reports the non-inferiority of baricitinib to adalimumab.
Business Wire. U.S. FDA issues complete response letter for baricitinib. http://www.businesswire.com/news/home/20170414005051/en/ Business Wire (2017).
Eli Lilly and Company. Lilly to file baricitinib resubmission to U. S. FDA before end of January 2018. Drugs.com https://www.drugs.com/nda/baricitinib_170830.html (2017).
Takeuchi, T. et al. Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: a 12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann. Rheum. Dis. 75, 1057–1064 (2016). This study reports the safety and efficacy of peficitinib in combination with methotrexate for the treatment of rheumatoid arthritis in methotrexate-intolerant patients.
Kivitz, A. J. et al. Peficitinib, a JAK inhibitor, in the treatment of moderate-to-severe rheumatoid arthritis in methotrexate-inadequate responders. Arthritis Rheumatol. 69, 709–719 (2017). This study reports the safety and efficacy of peficitinib monotherapy in patients with moderate to severe rheumatoid arthritis.
Genovese, M. C. et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 69, 932–942 (2017). This study reports the safety and efficacy of peficitinib in combination with DMARDs in patients with moderate to severe rheumatoid arthritis.
Mease, P. J. et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, or adalimumab in patients with active psoriatic arthritis and an inadequate response to conventional synthetic DMARDs: a randomized, placebo-controlled, phase 3 trial [abstract]. Arthritis Rheumatol. 68 (Suppl. 10), 2983 (2016). This study examines the effect of tofacitinib in psoriatic arthritis and was therefore important in the FDA's 2017 decision to recommend approval of tofacitinib for this condition.
Gao, W. et al. Tofacitinib regulates synovial inflammation in psoriatic arthritis, inhibiting STAT activation and induction of negative feedback inhibitors. Ann. Rheum. Dis. 75, 311–315 (2016).
van der Heijde, D. et al. Tofacitinib in patients with ankylosing spondylitis: a phase 2, 16-week, randomized, placebo-controlled, dose-ranging study [abstract]. Arthritis Rheumatol. 67 (Suppl. 10), 5L (2015).
van der Heijde, D. et al. Tofacitinib in patients with ankylosing spondylitis: a phase 2, 16-week, randomised, placebo-controlled, dose-ranging study [abstract]. Ann. Rheum. Dis. 75 (Suppl. 2), OP0002 (2016).
Tseng, B. et al. Tofacitinib response in juvenile idiopathic arthritis (JIA) and collagenous colitis. J. Clin. Rheumatol. 22, 446–448 (2016).
Sandborn, W. J. et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 376, 1723–1736 (2017). This paper contains findings from three separate clinical trials evaluating tofacitinib as both an induction and a maintenance therapy for moderate to severe ulcerative colitis. Tofacitinib demonstrated therapeutic efficacy in all three trials.
Sandborn, W. J. et al. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn's disease. Clin. Gastroenterol. Hepatol. 12, 1485–1493.e2 (2014).
Panés, J. et al. Tofacitinib for induction and maintenance therapy of Crohn's disease: results of two phase IIb randomised placebo-controlled trials. Gut 66, 1049–1059 (2017).
Nalleweg, N. et al. IL-9 and its receptor are predominantly involved in the pathogenesis of UC. Gut 64, 743–755 (2015).
Rutz, S., Wang, X. & Ouyang, W. The IL-20 subfamily of cytokines — from host defence to tissue homeostasis. Nat. Rev. Immunol. 14, 783–795 (2014).
Gerlach, K. et al. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat. Immunol. 15, 676–686 (2014).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012).
Leung, J. M. et al. IL-22-producing CD4+ cells are depleted in actively inflamed colitis tissue. Mucosal Immunol. 7, 124–133 (2014).
Casanova, J. L. & Abel, L. Revisiting Crohn's disease as a primary immunodeficiency of macrophages. J. Exp. Med. 206, 1839–1843 (2009).
Bissonnette, R. et al. Tofacitinib withdrawal and retreatment in moderate-to-severe chronic plaque psoriasis: a randomized controlled trial. Br. J. Dermatol. 172, 1395–1406 (2015).
Chiricozzi, A. et al. Tofacitinib for the treatment of moderate-to-severe psoriasis. Expert Rev. Clin. Immunol. 11, 443–455 (2015).
Krueger, J. et al. Tofacitinib attenuates pathologic immune pathways in patients with psoriasis: a randomized phase 2 study. J. Allergy Clin. Immunol. 137, 1079–1090 (2016).
Bachelez, H. et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet 386, 552–561 (2015). This study compares tofacitinib to etanercept or placebo in the treatment of psoriasis, finding that a 10 mg daily dose of tofacitinib is non-inferior to etanercept but that a 5 mg dose is inferior.
Reuters Staff. FDA declines to expand approval of Pfizer arthritis drug. Reuters http://www.reuters.com/article/pfizer-psoriasis-fda/fda-declines-to-expand-approval-of-pfizer-arthritis-drug-idUSL1N12E2OW20151014 (2015).
Papp, K. A. et al. A randomized phase 2b trial of baricitinib, an oral Janus kinase (JAK) 1/JAK2 inhibitor, in patients with moderate-to-severe psoriasis. Br. J. Dermatol. 174, 1266–1276 (2016).
Papp, K. et al. A phase 2a randomized, double-blind, placebo-controlled, sequential dose-escalation study to evaluate the efficacy and safety of ASP015K, a novel Janus kinase inhibitor, in patients with moderate-to-severe psoriasis. Br. J. Dermatol. 173, 767–776 (2015).
Papp, K. A. et al. Treatment of plaque psoriasis with an ointment formulation of the Janus kinase inhibitor, tofacitinib: a phase 2b randomized clinical trial. BMC Dermatol. 16, 15 (2016).
Punwani, N. et al. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. J. Am. Acad. Dermatol. 67, 658–664 (2012).
Punwani, N. et al. Downmodulation of key inflammatory cell markers with a topical Janus kinase 1/2 inhibitor. Br. J. Dermatol. 173, 989–997 (2015).
Guo, H., Cheng, Y., Shapiro, J. & McElwee, K. The role of lymphocytes in the development and treatment of alopecia areata. Expert Rev. Clin. Immunol. 11, 1335–1351 (2015).
Xing, L. et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 20, 1043–1049 (2014).
Liu, L. Y., Craiglow, B. G., Dai, F. & King, B. A. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J. Am. Acad. Dermatol. 76, 22–28 (2017). This 90-patient retrospective study reviews the efficacy of long-term tofacitinib use for the treatment of alopecia areata, finding that clinical response was seen in 77% of treated patients.
Craiglow, B. G., Liu, L. Y. & King, B. A. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J. Am. Acad. Dermatol. 76, 29–32 (2017).
Scheinberg, M. & Ferreira, S. B. Reversal of alopecia universalis by tofacitinib: a case report. Ann. Intern. Med. 165, 750–751 (2016).
Kennedy Crispin, M. et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight 1, e89776 (2016). This 3-month open-label study of 60 patients investigates tofacitinib for the treatment of alopecia areata, finding clinical efficacy in this condition.
Pieri, L., Guglielmelli, P. & Vannucchi, A. M. Ruxolitinib-induced reversal of alopecia universalis in a patient with essential thrombocythemia. Am. J. Hematol. 90, 82–83 (2015).
Jabbari, A. et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EBioMedicine 2, 351–355 (2015).
Gupta, A. K., Carviel, J. L. & Abramovits, W. Efficacy of tofacitinib in treatment of alopecia universalis in two patients. J. Eur. Acad. Dermatol. Venereol. 30, 1373–1378 (2016).
Anzengruber, F. et al. Transient efficacy of tofacitinib in alopecia areata universalis. Case Rep. Dermatol. 8, 102–106 (2016).
Craiglow, B. G., Tavares, D. & King, B. A. Topical ruxolitinib for the treatment of alopecia universalis. JAMA Dermatol. 152, 490–491 (2016).
Cosgrove, S. B. et al. A blinded, randomized, placebo-controlled trial of the efficacy and safety of the Janus kinase inhibitor oclacitinib (Apoquel®) in client-owned dogs with atopic dermatitis. Vet. Dermatol. 24, 587–597 (2013).
Gonzales, A. J. et al. Oclacitinib (APOQUEL®) is a novel Janus kinase inhibitor with activity against cytokines involved in allergy. J. Vet. Pharmacol. Ther. 37, 317–324 (2014).
Amano, W. et al. The Janus kinase inhibitor JTE-052 improves skin barrier function through suppressing signal transducer and activator of transcription 3 signaling. J. Allergy Clin. Immunol. 136, 667–677.e7 (2015).
Bao, L., Shi, V. Y. & Chan, L. S. IL-4 regulates chemokine CCL26 in keratinocytes through the Jak1, 2/Stat6 signal transduction pathway: implication for atopic dermatitis. Mol. Immunol. 50, 91–97 (2012).
Lee, H. Y., Stieger, M., Yawalkar, N. & Kakeda, M. Cytokines and chemokines in irritant contact dermatitis. Mediators Inflamm. 2013, 916497 (2013).
Amano, W. et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by downmodulating T cell activation and differentiation. J. Dermatol. Sci. 84, 258–265 (2016).
King, B., Lee, A. I. & Choi, J. Treatment of hypereosinophilic syndrome with cutaneous involvement with the JAK inhibitors tofacitinib and ruxolitinib. J. Invest. Dermatol. 137, 951–954 (2017).
Bissonnette, R. et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br. J. Dermatol. 175, 902–911 (2016).
Meng, J. & Steinhoff, M. Molecular mechanisms of pruritus. Curr. Res. Transl Med. 64, 203–206 (2016).
Craiglow, B. G. & King, B. A. Tofacitinib citrate for the treatment of vitiligo: a pathogenesis-directed therapy. JAMA Dermatol. 151, 1110–1112 (2015).
Koga, T. et al. Successful treatment of palmoplantar pustulosis with rheumatoid arthritis, with tofacitinib: impact of this JAK inhibitor on T-cell differentiation. Clin. Immunol. 173, 147–148 (2016).
Damsky, W. & King, B. A. Idiopathic erythema multiforme: evidence of underlying Janus kinase-signal transducer and activator of transcription activation and successful treatment with tofacitinib. JAAD Case Rep. 2, 502–504 (2016).
Wood, K. J., Bushell, A. & Hester, J. Regulatory immune cells in transplantation. Nat. Rev. Immunol. 12, 417–430 (2012).
Vincenti, F. et al. Randomized phase 2b trial of tofacitinib (CP-690,550) in de novo kidney transplant patients: efficacy, renal function and safety at 1 year. Am. J. Transplant. 12, 2446–2456 (2012).
Vincenti, F. et al. Evaluation of the effect of tofacitinib exposure on outcomes in kidney transplant patients. Am. J. Transplant. 15, 1644–1653 (2015).
Moore, C. A. et al. Janus kinase inhibition for immunosuppression in solid organ transplantation: is there a role in complex immunologic challenges? Hum. Immunol. 78, 64–71 (2016).
Montealegre, G. et al. Preliminary response to Janus kinase inhibition with baricitinib in chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperatures (CANDLE). Pediatr. Rheumatol. Online J. 13 (Suppl. 1), O31 (2015).
Montealegre Sanchez, G. A. et al. Lipodystrophy and elevated temperatures (CANDLE): clinical characterization and initial response to Janus kinase inhibition with baricitinib [abstract]. Arthritis Rheumatol. 65 (Suppl. 10), 1782 (2013).
Furumoto, Y. et al. Tofacitinib ameliorates murine lupus and its associated vascular dysfunction. Arthritis Rheumatol. 69, 148–160 (2017).
Paik, J. J. & Christopher-Stine, L. A case of refractory dermatomyositis responsive to tofacitinib. Semin. Arthritis Rheum. 46, e19 (2017).
Kurtzman, D. J. et al. Tofacitinib citrate for refractory cutaneous dermatomyositis: an alternative treatment. JAMA Dermatol. 152, 944–945 (2016).
Hornung, T. et al. Remission of recalcitrant dermatomyositis treated with ruxolitinib. N. Engl. J. Med. 371, 2537–2538 (2014).
[No authors listed.] The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 5, 75–92 (2007).
Liew, S. H. et al. Tofacitinib (CP-690,550), a Janus kinase inhibitor for dry eye disease: results from a phase 1/2 trial. Ophthalmology 119, 1328–1335 (2012).
Rothenberg, M. E. et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N. Engl. J. Med. 358, 1215–1228 (2008).
Rimar, D. et al. Tofacitinib for polyarteritis nodosa: a tailored therapy. Ann. Rheum. Dis. 75, 2214–2216 (2016).
Okiyama, N. et al. Reversal of CD8 T-cell-mediated mucocutaneous graft-versus-host-like disease by the JAK inhibitor tofacitinib. J. Invest. Dermatol. 134, 992–1000 (2014).
Martin, R., Sospedra, M., Rosito, M. & Engelhardt, B. Current multiple sclerosis treatments have improved our understanding of MS autoimmune pathogenesis. Eur. J. Immunol. 46, 2078–2090 (2016).
Tuttle, K. et al. Baricitinib in diabetic kidney disease: results from a phase 2, multi-center, randomized, double-blind, placebo-controlled study [abstract]. Diabetes 64 (Suppl. 1) a114-LB (2015).
Christ, A., Bekkering, S., Latz, E. & Riksen, N. P. Long-term activation of the innate immune system in atherosclerosis. Semin. Immunol. 28, 384–393 (2016).
Volpato, S. et al. Cardiovascular disease, interleukin-6, and risk of mortality in older women: the women's health and aging study. Circulation 103, 947–953 (2001).
Szelag, M., Piaszyk-Borychowska, A., Plens-Galaska, M., Wesoly, J. & Bluyssen, H. A. Targeted inhibition of STATs and IRFs as a potential treatment strategy in cardiovascular disease. Oncotarget 7, 48788–48812 (2016).
Dowty, M. E. et al. An analysis of in vitro cytokine inhibition profiles of tofacitinib and other Janus kinase inhibitors at clinically-meaningful concentrations [abstract]. Arthritis Rheumatol. 66 (Suppl. 10), 1514 (2014).
Strand, V. et al. Systematic review and meta-analysis of serious infections with tofacitinib and biologic disease-modifying antirheumatic drug treatment in rheumatoid arthritis clinical trials. Arthritis Res. Ther. 17, 362 (2015).
Wathes, R., Moule, S. & Milojkovic, D. Progressive multifocal leukoencephalopathy associated with ruxolitinib. N. Engl. J. Med. 369, 197–198 (2013).
Winthrop, K. L. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat. Rev. Rheumatol. 13, 234–243 (2017).
van Vollenhoven, R. et al. THU0199 Tofacitinib, an oral Janus kinase inhibitor, in the treatment of rheumatoid arthritis: changes in lymphocytes and lymphocyte subset counts and reversibility after up to 8 years of tofacitinib treatment [abstract]. Ann. Rheum. Dis. 75, 258 (2016).
Rizzi, M. et al. Impact of tofacitinib treatment on human B-cells in vitro and in vivo. J. Autoimmun. 77, 55–66 (2016).
Fink, K. Origin and function of circulating plasmablasts during acute viral infections. Front. Immunol. 3, 78 (2012).
Winthrop, K. L. et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann. Rheum. Dis. 75, 687–695 (2015).
Singh, J. A. et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 68, 1–26 (2016).
Winthrop, K. L. et al. The safety and immunogenicity of live zoster vaccination in rheumatoid arthritis patients before starting tofacitinib: a randomized phase II trial. Arthritis Rheumatol. http://dx.doi.org/10.1002/art.40187 (2017).
Winthrop, K. L. et al. Herpes zoster and tofacitinib: clinical outcomes and the risk of concomitant therapy. Arthritis Rheumatol. http://dx.doi.org/10.1002/art.40189 (2017).
Taylor, P. et al. Baricitinib versus placebo or adalimumab in patients with active rheumatoid arthritis (RA) and an inadequate response to background methotrexate therapy: results of a phase 3 study [abstract]. Arthritis Rheumatol. 67 (Suppl. 10), 2L (2015).
Solomon, D. H. et al. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol. 67, 1449–1455 (2015).
Alemao, E. et al. Cardiovascular risk factor management in patients with RA compared to matched non-RA patients. Rheumatology 55, 809–816 (2016).
Radner, H., Lesperance, T., Accortt, N. A. & Solomon, D. H. Incidence and prevalence of cardiovascular risk factors among patients with rheumatoid arthritis, psoriasis, or psoriatic arthritis. Arthritis Care Res. http://dx.doi.org/10.1002/acr.23171 (2016).
Mok, C. C. et al. Prevalence of atherosclerotic risk factors and the metabolic syndrome in patients with chronic inflammatory arthritis. Arthritis Care Res. 63, 195–202 (2011).
Hak, A. E., Karlson, E. W., Feskanich, D., Stampfer, M. J. & Costenbader, K. H. Systemic lupus erythematosus and the risk of cardiovascular disease: results from the nurses' health study. Arthritis Rheum. 61, 1396–1402 (2009).
Tobin, A. M. et al. Cardiovascular disease and risk factors in patients with psoriasis and psoriatic arthritis. J. Rheumatol. 37, 1386–1394 (2010).
Denny, M. F. et al. Interferon-alpha promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood 110, 2907–2915 (2007).
Scheller, J., Garbers, C. & Rose-John, S. Interleukin-6: from basic biology to selective blockade of pro-inflammatory activities. Semin. Immunol. 26, 2–12 (2014).
Souto, A. et al. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: a systematic review and meta-analysis. Arthritis Rheumatol. 67, 117–127 (2015).
Rao, V. U. et al. An evaluation of risk factors for major adverse cardiovascular events during tocilizumab therapy. Arthritis Rheumatol. 67, 372–380 (2015).
Charles-Schoeman, C. et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol. 67, 616–625 (2015).
Charles-Schoeman, C. et al. Cardiovascular safety findings in patients with rheumatoid arthritis treated with tofacitinib, an oral Janus kinase inhibitor. Semin. Arthritis Rheum. 46, 261–271 (2016).
Wu, J. J. et al. Effects of tofacitinib on cardiovascular risk factors and cardiovascular outcomes based on phase III and long-term extension data in patients with plaque psoriasis. J. Am. Acad. Dermatol. 75, 897–905 (2016).
Charles-Schoeman, C. et al. Effects of tofacitinib and other DMARDs on lipid profiles in rheumatoid arthritis: implications for the rheumatologist. Semin. Arthritis Rheum. 46, 71–80 (2016).
Kume, K. et al. Tofacitinib improves arterial stiffness despite up-regulating serum cholesterol with chronic cardiovascular disease in methotrexate-resistant active rheumatoid arthritis patients. a cohort study [abstract]. Arthritis Rheumatol. 66 (Suppl. 10), 486 (2014).
Xie, F., Yun, H., Bernatsky, S. & Curtis, J. R. Brief report: risk of gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologic treatments. Arthritis Rheumatol. 68, 2612–2617 (2016).
Dudakov, J. A., Hanash, A. M. & van den Brink, M. R. Interleukin-22: immunobiology and pathology. Annu. Rev. Immunol. 33, 747–785 (2015).
Shouval, D. S. et al. Interleukin 10 receptor signaling: master regulator of intestinal mucosal homeostasis in mice and humans. Adv. Immunol. 122, 177–210 (2014).
Sivaraman, P. & Cohen, S. B. Malignancy and Janus kinase inhibition. Rheum. Dis. Clin. North Am. 43, 79–93 (2017).
Kremer, J. M. et al. Evaluation of the effect of tofacitinib on measured glomerular filtration rate in patients with active rheumatoid arthritis: results from a randomised controlled trial. Arthritis Res. Ther. 17, 95 (2015).
Isaacs, J. D. et al. Changes in serum creatinine in patients with active rheumatoid arthritis treated with tofacitinib: results from clinical trials. Arthritis Res. Ther. 16, R158 (2014).
Chapin, R. E. et al. Effects of the Janus kinase inhibitor, tofacitinib, on testicular Leydig cell hyperplasia and adenoma in rats, and on prolactin signaling in cultured primary rat Leydig cells. Toxicol. Sci. 155, 148–156 (2017).
Van Rompaey, L. et al. Preclinical characterization of GLPG0634, a selective inhibitor of JAK1, for the treatment of inflammatory diseases. J. Immunol. 191, 3568–3577 (2013).
Menet, C. J. et al. Triazolopyridines as selective JAK1 inhibitors: from hit identification to GLPG0634. J. Med. Chem. 57, 9323–9342 (2014).
Kettle, J. G. et al. Inhibitors of JAK-family kinases: an update on the patent literature 2013–2015, part 1. Expert Opin. Ther. Pat. 27, 127–143 (2016).
Kettle, J. G. et al. Inhibitors of JAK-family kinases: an update on the patent literature 2013–2015, part 2. Expert Opin. Ther. Pat. 27, 145–161 (2017).
Shimozaki, K., Nakajima, K., Hirano, T. & Nagata, S. Involvement of STAT3 in the granulocyte colony-stimulating factor-induced differentiation of myeloid cells. J. Biol. Chem. 272, 25184–25189 (1997).
Vermeire, S. et al. Clinical remission in patients with moderate-to-severe Crohn's disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 389, 266–275 (2017). This study tests the selective jakinib filgotinib in the treatment of moderate to severe Crohn's disease, finding it to be significantly effective at inducing clinical remission when compared with placebo.
Voss, J. et al. Pharmacodynamics of a novel JAK1 selective inhibitor in rat arthritis and anemia models and in healthy human subjects [abstract]. Ann. Rheum. Dis. 73 (Suppl. 2), THU0127 (2014).
Graff, C. et al. Characterization of ABT-494, a second generation Jak1 selective inhibitor [abstract]. Arthritis Rheumatol. 66 (Suppl. 10), 1499 (2014).
Kremer, J. M. et al. A phase IIb study of ABT-494, a selective JAK-1 inhibitor, in patients with rheumatoid arthritis and an inadequate response to anti-tumor necrosis factor therapy. Arthritis Rheumatol. 68, 2867–2877 (2016).
Genovese, M. C. et al. Efficacy and safety of ABT-494, a selective JAK-1 inhibitor, in a phase IIb study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol. 68, 2857–2866 (2016). This trial finds upadacitinib to be effective in patients with rheumatoid arthritis who had not responded to methotrexate.
Mohamed, M. E., Jungerwirth, S., Asatryan, A., Jiang, P. & Othman, A. Assessment of the effect of CYP3A inhibition, CYP induction, OATP1B inhibition and administration of high-fat meal on the pharmacokinetics of the potent and selective JAK1 inhibitor ABT-494 [abstract]. Arthritis Rheumatol. 67 (Suppl. 10), 2751 (2015).
Eichelbaum, M. & Burk, O. CYP3A genetics in drug metabolism. Nat. Med. 7, 285–287 (2001).
Henriques, C. AbbVie launches phase 3 trial for rheumatoid arthritis. Rheumatoid Arthritis News http://rheumatoidarthritisnews.com/2016/01/29/abbvie-announces-the-launch-of-robust-phase-3-clinical-trial-program-evaluating-abt-494-an-investigational-selective-jak1-inhibitor-for-the-treatment-of-rheumatoid-arthritis-2/ (2016).
Sandborn, W. J. et al. Safety and efficacy of ABT-494 (upadacitinib), an oral Jak1 Inhibitor, as induction therapy in patients with Crohn's disease: results from Celest [abstract]. Gastroenterology 152 (Suppl. 1), 874h (2017). This abstract contains results from the CELEST trial, which reported that upadacitinib is safe and efficacious in the treatment of Crohn's disease.
Ludbrook, V. J. et al. Investigation of selective JAK1 inhibitor GSK2586184 for the treatment of psoriasis in a randomized placebo-controlled phase IIa study. Br. J. Dermatol. 174, 985–995 (2016).
Kahl, L. et al. Safety, tolerability, efficacy and pharmacodynamics of the selective JAK1 inhibitor GSK2586184 in patients with systemic lupus erythematosus. Lupus 25, 1420–1430 (2016). This trial finds decernotinib monotherapy to be efficacious in the treatment of rheumatoid arthritis.
Clark, J. D., Flanagan, M. E. & Telliez, J. B. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J. Med. Chem. 57, 5023–5038 (2014). This trial finds decernotinib in combination with conventional DMARDs to be efficacious in the treatment of rheumatoid arthritis.
Genovese, M. C., van Vollenhoven, R. F., Pacheco-Tena, C., Zhang, Y. & Kinnman, N. VX-509 (decernotinib), an oral selective JAK-3 inhibitor, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheumatol. 68, 46–55 (2016).
Genovese, M. C., Yang, F., Ostergaard, M. & Kinnman, N. Efficacy of VX-509 (decernotinib) in combination with a disease-modifying antirheumatic drug in patients with rheumatoid arthritis: clinical and MRI findings. Ann. Rheum. Dis. 75, 1979–1983 (2016).
Farmer, L. J. et al. Discovery of VX-509 (decernotinib): a potent and selective Janus kinase 3 inhibitor for the treatment of autoimmune diseases. J. Med. Chem. 58, 7195–7216 (2015).
Fleischmann, R. M. et al. A randomized, double-blind, placebo-controlled, twelve-week, dose-ranging study of decernotinib, an oral selective JAK-3 inhibitor, as monotherapy in patients with active rheumatoid arthritis. Arthritis Rheumatol 67, 334–343 (2015).
Huang, J., Yang, F., Yogaratnam, J. & Shen, J. The effect of deuteration of VX-509 (decernotinib) on drug-drug interactions (DDI) with midazolam [abstract]. Ann. Rheum. Dis. 74 (Suppl. 2), SAT0227 (2015).
Goedken, E. R. et al. Tricyclic covalent inhibitors selectively target Jak3 through an active site thiol. J. Biol. Chem. 290, 4573–4589 (2015).
Telliez, J. B. et al. Discovery of a JAK3-selective inhibitor: functional differentiation of JAK3-selective inhibition over pan-JAK or JAK1-selective inhibition. ACS Chem. Biol. 11, 3442–3451 (2016).
Miao, W., Masse, C., Greenwood, J., Kapeller, R. & Westlin, W. Potent and selective Tyk2 inhibitor highly efficacious in rodent models of inflammatory bowel disease and psoriasis [abstract]. Arthritis Rheumatol. 68 (Suppl. 10), 1911 (2016).
Masse, C. et al. Identification of highly potent and selective Tyk2 inhibitors for the treatment of autoimmune diseases through structure-based drug design (THER2P.961). J. Immunol. 194, 67 (2015).
Gillooly, K. et al. BMS-986165 is a highly potent and selective allosteric inhibitor of Tyk2, blocks IL-12, IL-23 and type I interferon signaling and provides for robust efficacy in preclinical models of systemic lupus erythematosus and inflammatory bowel disease [abstract]. Arthritis Rheumatol. 68 (Suppl. 10), 11L (2016).
Moslin, R. et al. Pseudokinase domain of the Janus Kinase (JAK) TYK2: a novel target for the treatment of immune-mediated inflammatory diseases in 25th Enzyme Mechanisms Conference, O4 (St. Pete Beach, 2017).
Fensome, A. et al. in 252nd American Chemical Society 2016 Annual Meeting and Expo, MEDI 271 (Philadelphia, 2016).
Works, M. G. et al. Inhibition of TYK2 and JAK1 ameliorates imiquimod-induced psoriasis-like dermatitis by inhibiting IL-22 and the IL-23/IL-17 axis. J. Immunol. 193, 3278–3287 (2014).
Mocsai, A., Ruland, J. & Tybulewicz, V. L. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat. Rev. Immunol. 10, 387–402 (2010).
Weinblatt, M. E. et al. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. N. Engl. J. Med. 363, 1303–1312 (2010).
Kheirkhah, A. et al. A pilot randomized trial on safety and efficacy of a novel topical combined inhibitor of Janus kinase 1/3 and spleen tyrosine kinase for GVHD-associated ocular surface disease. Cornea 36, 799–804 (2017).
Coffey, G. et al. The novel kinase inhibitor PRT062070 (Cerdulatinib) demonstrates efficacy in models of autoimmunity and B-cell cancer. J. Pharmacol. Exp. Ther. 351, 538–548 (2014).
Hume, D. A. & MacDonald, K. P. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 119, 1810–1820 (2012).
Kindler, T., Lipka, D. B. & Fischer, T. FLT3 as a therapeutic target in AML: still challenging after all these years. Blood 116, 5089–5102 (2010).
Madan, B. et al. SB1578, a novel inhibitor of JAK2, FLT3, and c-Fms for the treatment of rheumatoid arthritis. J. Immunol. 189, 4123–4134 (2012).
William, A. D. et al. Discovery of the macrocycle (9E)-15-(2-(pyrrolidin-1-yl)ethoxy)-7,12,25-trioxa-19,21,24-triaza-tetracyclo[18.3.1.1(2,5).1(14,18)]hexacosa-1(24),2,4,9,14(26),15,17,20,22-nonaene (SB1578), a potent inhibitor of Janus kinase 2/FMS-like tyrosine kinase-3 (JAK2/FLT3) for the treatment of rheumatoid arthritis. J. Med. Chem. 55, 2623–2640 (2012).
Smith, G. A., Uchida, K., Weiss, A. & Taunton, J. Essential biphasic role for JAK3 catalytic activity in IL-2 receptor signaling. Nat. Chem. Biol. 12, 373–379 (2016).
Haan, C. et al. Jak1 has a dominant role over Jak3 in signal transduction through γc-containing cytokine receptors. Chem. Biol. 18, 314–323 (2011).
Karaman, M. W. et al. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. 26, 127–132 (2008).
Li, L. et al. Beryllium-induced lung disease exhibits expression profiles similar to sarcoidosis. Eur. Respir. J. 47, 1797–1808 (2016).
Moodley, D. et al. Network pharmacology of JAK inhibitors. Proc. Natl Acad. Sci. USA 113, 9852–9857 (2016).
Barroso, N. & Furst, D. E. A. Case series on patients on tofacitinib in combination with a biologic [abstract]. Arthritis Rheumatol. 68 (Suppl. 10), 1651 (2016).
Vahedi, G. et al. Super-enhancers delineate disease-associated regulatory nodes in T cells. Nature 520, 558–562 (2015).
McNally, R. & Eck, M. J. JAK-cytokine receptor recognition, unboxed. Nat. Struct. Mol. Biol. 21, 431–433 (2014). This study examines the effect of tofacitinib in psoriatic arthritis and was therefore important in the FDA's 2017 decision to recommend approval of tofacitinib for this condition.
Chen, X. et al. Crystal structure of a tyrosine phosphorylated STAT-1 dimer bound to DNA. Cell 93, 827–839 (1998).
Acknowledgements
The work of D.M.S., M.G. and J.O'S. is supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the US National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J.O'S. declares that he and the US Government receive royalties based on patents related to the targeting of Janus kinases. J.O'S., M.G. and the US Government have had longstanding Cooperative Research and Development Agreements with Pfizer, which produces tofacitinib, a Janus kinase inhibitor.
Glossary
- C-reactive protein
-
(CRP). A protein classified as an acute phase reactant and produced in the liver in response to inflammation.
- Serum amyloid A
-
An acute phase reactant, produced predominantly in the liver and expressed at different levels in response to inflammatory stimuli.
- Phosphoproteomic analysis
-
A proteome-wide analysis of phosphorylated proteins.
- American College of Rheumatology 20%, 50% and 70% response criteria
-
(ACR20, 50, 70). Standard criteria used to measure the effectiveness of treatments for rheumatoid arthritis. These criteria measure percentage improvement in tender or swollen joint counts and three of the following measures: patient assessment; physician assessment; pain scale; disability/functional questionnaire and acute phase reactants
- 36-Item Short Form Survey
-
(SF-36). A set of generic, coherent and easily administered quality-of-life measures that rely on patient self-reporting and are now widely used by managed care organizations for routine monitoring and assessment of care outcomes in adult patients.
- Biologics
-
Agents that target cytokines or cytokine receptors, usually monoclonal antibodies or chimeric receptors.
- Baseline structural damage
-
Abnormal imaging findings on radiographic assessment, specifically joint space narrowing and erosion.
- Complete response letter
-
A letter issued by the US Food and Drug Administration to communicate that it has completed its review of a drug application and decided not to approve it in its present form.
- Transmural inflammation
-
Full-thickness inflammation across the entire bowel wall (as opposed to being limited to the mucosa and submucosa).
- Skip lesions
-
Lesions that are discontinuous. In the context of this Review, the term refers to discontinuous lesions in the gastrointestinal tract.
- Psoriasis Activity and Severity Index
-
A standardized score used to measure the severity and extent of skin involvement in psoriasis. A representative area of psoriasis is selected for each body region, and the intensity of redness, thickness and scaliness is assessed on a scale of 0 (none) to 4 (very severe).
- BK viraemia
-
Disseminated viral infection with BK virus, a polyomavirus whose name is an abbreviation of the name of the index patient from whom this virus was isolated.
- Post-transplant lymphoproliferative disease
-
(PTLD). A post-transplantation malignancy that can occur as a complication of solid organ or haematopoietic stem cell transplantation. Often associated with Epstein-Barr virus infection of B cells.
- Sjogren's syndrome
-
A systemic autoimmune condition characterized by autoimmune exocrinopathy (salivary and lacrimal glands), as well as, in some cases, systemic inflammation (central nervous system, hepatic, skin, renal and others).
- Myositis
-
Inflammation of the muscles. In the context of this Review, myositis refers to the two systemic autoimmune conditions polymyositis and dermatomyositis.
- Eosinophilic oesophagitis
-
A disease characterized by eosinophilic inflammation of the oesophagus, also termed allergic oesophagitis.
- Plasmablasts
-
Immature cells of plasma cell lineage, a type of B cell. Plasmablasts secrete more antibodies than B cells but fewer than mature plasma cells.
Rights and permissions
About this article
Cite this article
Schwartz, D., Kanno, Y., Villarino, A. et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov 16, 843–862 (2017). https://doi.org/10.1038/nrd.2017.201
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2017.201
This article is cited by
-
Single-cell RNA sequencing reveals roles of unique retinal microglia types in early diabetic retinopathy
Diabetology & Metabolic Syndrome (2024)
-
IL-17 in wound repair: bridging acute and chronic responses
Cell Communication and Signaling (2024)
-
Tofacitinib for the treatment of immune-related adverse events in cancer immunotherapy: a multi-center observational study
Journal of Translational Medicine (2024)
-
Januskinaseinhibitoren
Die Dermatologie (2024)
-
A Review on Nanosystem-Based Delivery of Tofacitinib for Enhanced Treatment of Autoimmune Diseases and Inflammation
BioNanoScience (2024)