Key Points
-
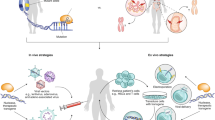
Genome-editing systems have remarkable potential to treat genetic diseases. However, one of the major challenges facing their implementation is the safe and efficient intracellular delivery of genome-editing biomacromolecules, including nucleases and nucleic acids.
-
Nanoparticles encapsulating genome-editing biomacromolecules must be endocytosed by the cell of interest and reach the nucleus or cytoplasm to function; additional extracellular barriers are present for in vivo delivery.
-
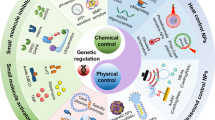
Physical methods for in vitro and ex vivo delivery of genome-editing tools include electroporation, membrane deformation and microinjection.
-
Viral vectors are widely used to deliver DNA for genome editing and are typically integrase-defective lentiviral vectors (IDLVs), adenoviruses and adeno-associated viruses (AAVs); AAVs seem to be the most popular vector for in vivo applications.
-
Non-viral nanoparticles, most often made from synthetic and cationic lipid or polymer delivery materials, can be used to deliver genome-editing tools in vitro, ex vivo and in vivo, sometimes in combination with viral vectors.
-
At the time of writing, the genome-editing industry is in its infancy and includes ongoing and completed phase I and phase II clinical trials.
-
In addition to selecting an effective delivery material, scientists must consider safety (that is, off-target effects, immunogenicity and mutagenesis), the required amount of genomic modification for therapeutic benefit, and the duration of effects.
Abstract
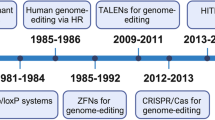
With the recent development of CRISPR technology, it is becoming increasingly easy to engineer the genome. Genome-editing systems based on CRISPR, as well as transcription activator-like effector nucleases (TALENs) and zinc-finger nucleases (ZFNs), are becoming valuable tools for biomedical research, drug discovery and development, and even gene therapy. However, for each of these systems to effectively enter cells of interest and perform their function, efficient and safe delivery technologies are needed. This Review discusses the principles of biomacromolecule delivery and gene editing, examines recent advances and challenges in non-viral and viral delivery methods, and highlights the status of related clinical trials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Boycott, K. M., Vanstone, M. R., Bulman, D. E. & MacKenzie, A. E. Rare-disease genetics in the era of next-generation sequencing: discovery to translation. Nat. Rev. Genet. 14, 681–691 (2013).
Pharmaceutical Research and Manufacturers of America. Medicines In Development. Rare Diseases: A Report On Orphan Drugs In The Pipeline Presented By America's Biopharmaceutical Research Companies. PhRMA.org http://phrma-docs.phrma.org/sites/default/files/pdf/Rare_Diseases_2013.pdf (2013).
Elborn, J. S. Cystic fibrosis. Lancet 388, 2519–2531 (2016).
Yang, Q. Small molecule therapy for genetic diseases. Yale J. Biol. Med. 85, 161–162 (2012).
O'Connor, T. P. & Crystal, R. G. Genetic medicines: treatment strategies for hereditary disorders. Nat. Rev. Genet. 7, 261–276 (2006).
Winkel, L. P. et al. Enzyme replacement therapy in late-onset Pompe's disease: a three-year follow-up. Ann. Neurol. 55, 495–502 (2004).
Srivastava, A. Dose and response in haemophilia — optimization of factor replacement therapy. Br. J. Haematol. 127, 12–25 (2004).
Holz, F. G., Schmitz-Valckenberg, S. & Fleckenstein, M. Recent developments in the treatment of age-related macular degeneration. J. Clin. Invest. 124, 1430–1438 (2014).
Gorzelany, J. A. & de Souza, M. P. Protein replacement therapies for rare diseases: a breeze for regulatory approval? Sci. Transl Med. 5, 178fs10 (2013).
Yin, H. et al. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 15, 541–555 (2014).
Kay, M. A. State-of-the-art gene-based therapies: the road ahead. Nat. Rev. Genet. 12, 316–328 (2011).
Mansoor, M. & Melendez, A. J. Advances in antisense oligonucleotide development for target identification, validation, and as novel therapeutics. Gene Regul. Syst. Bio. 2, 275–295 (2008).
Castanotto, D. & Rossi, J. J. The promises and pitfalls of RNA-interference-based therapeutics. Nature 457, 426–433 (2009).
Baum, C., Kustikova, O., Modlich, U., Li, Z. & Fehse, B. Mutagenesis and oncogenesis by chromosomal insertion of gene transfer vectors. Hum. Gene Ther. 17, 253–263 (2006).
Herzog, R. W. Hemophilia gene therapy: caught between a cure and an immune response. Mol. Ther. 23, 1411–1412 (2015).
Herzog, R. W., Davidoff, A. M., Markusic, D. M. & Nathwani, A. C. AAV vector biology in primates: finding the missing link? Mol. Ther. 19, 1923–1924 (2011).
Wang, L. et al. AAV8-mediated hepatic gene transfer in infant rhesus monkeys (Macaca mulatta). Mol. Ther. 19, 2012–2020 (2011).
Naldini, L. Gene therapy returns to centre stage. Nature 526, 351–360 (2015).
Doudna, J. A. & Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096 (2014).
Stoddard, B. L. Homing endonucleases: from microbial genetic invaders to reagents for targeted DNA modification. Structure 19, 7–15 (2011).
Urnov, F. D., Rebar, E. J., Holmes, M. C., Zhang, H. S. & Gregory, P. D. Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 11, 636–646 (2010).
Joung, J. K. & Sander, J. D. TALENs: a widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 14, 49–55 (2013).
Rouet, P., Smih, F. & Jasin, M. Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol. Cell. Biol. 14, 8096–8106 (1994).
Lieber, M. R., Ma, Y., Pannicke, U. & Schwarz, K. Mechanism and regulation of human non-homologous DNA end-joining. Nat. Rev. Mol. Cell Biol. 4, 712–720 (2003).
Isken, O. & Maquat, L. E. Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev. 21, 1833–1856 (2007).
Long, C. et al. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 351, 400–403 (2016).
Long, C. et al. Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science 345, 1184–1188 (2014).
Nelson, C. E. et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351, 403–407 (2016).
Tabebordbar, M. et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 351, 407–411 (2016).
Xu, L. et al. CRISPR-mediated genome editing restores dystrophin expression and function in mdx mice. Mol. Ther. 24, 564–569 (2016). References 26, 28, 29 and 30 demonstrate the use of viral vectors to correct disease-causing mutations in a mouse model of Duchenne muscular dystrophy.
Sadelain, M., Papapetrou, E. P. & Bushman, F. D. Safe harbours for the integration of new DNA in the human genome. Nat. Rev. Cancer 12, 51–58 (2012).
Li, H. et al. In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature 475, 217–221 (2011). This study is the first to demonstrate that ZFN-mediated gene correction can be achieved in vivo and reverse the disease phenotype.
Yin, H. et al. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat. Biotechnol. 32, 551–553 (2014). This study is the first time to demonstrate that CRISPR can correct a disease mutation in vivo and reverse disease symptoms.
Oakes, B. L. et al. Multi-reporter selection for the design of active and more specific zinc-finger nucleases for genome editing. Nat. Commun. 7, 10194 (2016).
Bhakta, M. S. et al. Highly active zinc-finger nucleases by extended modular assembly. Genome Res. 23, 530–538 (2013).
Wilson, K. A. et al. Expanding the repertoire of target sites for zinc finger nuclease-mediated genome modification. Mol. Ther. Nucleic Acids 2, e88 (2013).
Shukla, V. K. et al. Precise genome modification in the crop species Zea mays using zinc-finger nucleases. Nature 459, 437–441 (2009).
Kim, H. & Kim, J.-S. A guide to genome engineering with programmable nucleases. Nat. Rev. Genet. 15, 321–334 (2014).
Kim, Y. et al. A library of TAL effector nucleases spanning the human genome. Nat. Biotechnol. 31, 251–258 (2013).
Lin, S., Staahl, B. T., Alla, R. K. & Doudna, J. A. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. eLife 3, e04766 (2015).
Hsu, P. D. et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31, 827–832 (2013).
Gabriel, R. et al. An unbiased genome-wide analysis of zinc-finger nuclease specificity. Nat. Biotechnol. 29, 816–823 (2011).
Sander, J. D. et al. In silico abstraction of zinc finger nuclease cleavage profiles reveals an expanded landscape of off-target sites. Nucleic Acids Res. 41, e181 (2013).
Tsai, S. Q. et al. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat. Biotechnol. 33, 187–197 (2015).
Frock, R. L. et al. Genome-wide detection of DNA double-stranded breaks induced by engineered nucleases. Nat. Biotechnol. 33, 179–186 (2015).
Kim, D. et al. Digenome-seq: genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. Nat. Methods 12, 237–243 (2015).
Wang, X. et al. Unbiased detection of off-target cleavage by CRISPR-Cas9 and TALENs using integrase-defective lentiviral vectors. Nat. Biotechnol. 33, 175–178 (2015).
Ran, F. A. et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154, 1380–1389 (2013).
Mali, P. et al. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol. 31, 833–838 (2013).
Tsai, S. Q. et al. Dimeric CRISPR RNA-guided FokI nucleases for highly specific genome editing. Nat. Biotechnol. 32, 569–576 (2014).
Guilinger, J. P., Thompson, D. B. & Liu, D. R. Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification. Nat. Biotechnol. 32, 577–582 (2014).
Fu, Y., Sander, J. D., Reyon, D., Cascio, V. M. & Joung, J. K. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat. Biotechnol. 32, 279–284 (2013).
Slaymaker, I. M. et al. Rationally engineered Cas9 nucleases with improved specificity. Science 351, 84–88 (2016).
Kleinstiver, B. P. et al. High-fidelity CRISPR-Cas9 nucleases with no detec table genome-wide off-target effects. Nature 529, 490–495 (2016).
Bolukbasi, M. F. et al. DNA-binding-domain fusions enhance the targeting range and precision of Cas9. Nat. Methods 12, 1150–1156 (2015)
Sollu, C. et al. Autonomous zinc-finger nuclease pairs for targeted chromosomal deletion. Nucleic Acids Res. 38, 8269–8276 (2010).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Yang, H. et al. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell 154, 1370–1379 (2013).
Senis, E. et al. CRISPR/Cas9-mediated genome engineering: an adeno-associated viral (AAV) vector toolbox. Biotechnol. J. 9, 1402–1412 (2014).
Genovese, P. et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature 510, 235–240 (2014). This study demonstrates that gene-edited HSCs can sustain normal haematopoiesis.
Wang, D. et al. Adenovirus-mediated somatic genome editing of Pten by CRISPR/Cas9 in mouse liver in spite of Cas9-specific immune responses. Hum. Gene Ther. 26, 432–442 (2015).
Schumann, K. et al. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc. Natl Acad. Sci. USA 112, 10437–10442 (2015).
Mandal, P. K. et al. Efficient ablation of genes in human hematopoietic stem and effector cells using CRISPR/Cas9. Cell Stem Cell 15, 643–652 (2014).
Han, X. et al. CRISPR–Cas9 delivery to hard-to-transfect cells via membrane deformation. Sci. Adv. 1, e1500454 (2015).
Sharei, A. et al. A vector-free microfluidic platform for intracellular delivery. Proc. Natl Acad. Sci. USA 110, 2082–2087 (2013).
Zuris, J. A. et al. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol. 33, 73–80 (2014). This study reports that proteins fused to negatively charged domains can be intracellularly delivered by cationic lipids.
Liang, X. et al. Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection. J. Biotechnol. 208, 44–53 (2015).
Ramakrishna, S. et al. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res. 24, 1020–1027 (2014).
Sun, W. et al. Self-assembled DNA nanoclews for the efficient delivery of CRISPR-Cas9 for genome editing. Angew. Chem. Int. Ed. 54, 12029–12033 (2015).
Morris, E. C. & Stauss, H. J. Optimizing T cell receptor gene therapy for hematologic malignancies. Blood 127, 3305–3311 (2016).
Braun, C. J. et al. Gene therapy for Wiskott–Aldrich syndrome — long-term efficacy and genotoxicity. Sci. Transl Med. 6, 227ra33 (2014).
Bankiewicz, K. S. et al. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol. Ther. 14, 564–570 (2006).
Yin, H. et al. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat. Biotechnol. 34, 328–333 (2016). This study reports that a combination of lipid nanoparticles encapsulating Cas9 mRNA with an AAV encoding a repair donor and an sgRNA induces efficient repair of a disease gene in vivo.
Yin, H. et al. RNAi-nanoparticulate manipulation of gene expression as a new functional genomics tool in the liver. J. Hepatol. 64, 899–907 (2016).
Swiech, L. et al. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat. Biotechnol. 33, 102–106 (2014).
Lombardo, A. et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat. Biotechnol. 25, 1298–1306 (2007).
De Ravin, S. S. et al. Targeted gene addition in human CD34 hematopoietic cells for correction of X-linked chronic granulomatous disease. Nat. Biotechnol. 34, 424–429 (2016).
Wang, J. et al. Homology-driven genome editing in hematopoietic stem and progenitor cells using ZFN mRNA and AAV6 donors. Nat. Biotechnol. 33, 1256–1263 (2015).
Provasi, E. et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat. Med. 18, 807–815 (2012).
Sather, B. D. et al. Efficient modification of CCR5 in primary human hematopoietic cells using a megaTAL nuclease and AAV donor template. Sci. Transl Med. 7, 307ra156 (2015).
Holkers, M. et al. Adenoviral vector DNA for accurate genome editing with engineered nucleases. Nat. Methods 11, 1051–1057 (2014).
Weissman, I. L. & Shizuru, J. A. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood 112, 3543–3553 (2008).
Khalil, D. N., Smith, E. L., Brentjens, R. J. & Wolchok, J. D. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 13, 273–290 (2016).
DeKelver, R. C. et al. Functional genomics, proteomics, and regulatory DNA analysis in isogenic settings using zinc finger nuclease-driven transgenesis into a safe harbor locus in the human genome. Genome Res. 20, 1133–1142 (2010).
Cohen, C. J. et al. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 67, 3898–3903 (2007).
Beane, J. D. et al. Clinical scale zinc finger nuclease-mediated gene editing of PD-1 in tumor infiltrating lymphocytes for the treatment of metastatic melanoma. Mol. Ther. 23, 1380–1390 (2015).
Torikai, H. et al. Toward eliminating HLA class I expression to generate universal cells from allogeneic donors. Blood 122, 1341–1349 (2013).
Frederickson, R. M. A new era of innovation for CAR T-cell therapy. Mol. Ther. 23, 1795–1796 (2015).
Wang, J. et al. Highly efficient homology-driven genome editing in human T cells by combining zinc-finger nuclease mRNA and AAV6 donor delivery. Nucleic Acids Res. 44, e30 (2016).
Wang, X. et al. CRISPR-Cas9 targeting of PCSK9 in human hepatocytes in vivo. Arterioscler. Thromb. Vasc. Biol. 36, 783–786 (2016).
Ding, Q. et al. Permanent alteration of PCSK9 with in vivo CRISPR-Cas9 genome editing. Circ. Res. 115, 488–492 (2014).
Anguela, X. M. et al. Robust ZFN-mediated genome editing in adult hemophilic mice. Blood 122, 3283–3287 (2013).
Sharma, R. et al. In vivo genome editing of the albumin locus as a platform for protein replacement therapy. Blood 126, 1777–1784 (2015).
Zetsche, B., Volz, S. E. & Zhang, F. A split-Cas9 architecture for inducible genome editing and transcription modulation. Nat. Biotechnol. 33, 139–142 (2015).
Wright, A. V. et al. Rational design of a split-Cas9 enzyme complex. Proc. Natl Acad. Sci. USA 112, 2984–2989 (2015).
Ran, F. A. et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015). This study characterizes smaller Cas9 orthologues, and packages one orthologue and a guide RNA into a single AAV vector to perform in vivo editing.
Yang, Y. et al. A dual AAV system enables the Cas9-mediated correction of a metabolic liver disease in newborn mice. Nat. Biotechnol. 34, 334–338 (2016). This study uses dual AAV vectors to deliver Cas9, a guide RNA and a template DNA to efficiently correct a mutation in the liver.
Rols, M. P. Mechanism by which electroporation mediates DNA migration and entry into cells and targeted tissues. Methods Mol. Biol. 423, 19–33 (2008).
Kim, S., Kim, D., Cho, S. W., Kim, J. & Kim, J. S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 24, 1012–1019 (2014).
Hendel, A. et al. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat. Biotechnol. 33, 985–989 (2015). This study reports that chemical modification of sgRNA enhances genome-editing efficiency in primary cells and stem cells.
Derdelinckx, J., Berneman, Z. N. & Cools, N. GMP-grade mRNA electroporation of dendritic cells for clinical use. Methods Mol. Biol. 1428, 139–150 (2016).
DiTommaso, T., Gilbert, J., Bernstein, H. & Sharei, A. Vector free genome editing of immune cells for cell therapy. Mol. Ther. 24 (Suppl. 1), S229 (2016).
D'Astolfo, D. S. et al. Efficient intracellular delivery of native proteins. Cell 161, 674–690 (2015).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Schwank, G. et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13, 653–658 (2013).
Liu, J., Gaj, T., Patterson, J. T., Sirk, S. J. & Barbas, C. F. III. Cell-penetrating peptide-mediated delivery of TALEN proteins via bioconjugation for genome engineering. PLoS ONE 9, e85755 (2014).
Gaj, T., Guo, J., Kato, Y., Sirk, S. J. & Barbas, C. F. III. Targeted gene knockout by direct delivery of zinc-finger nuclease proteins. Nat. Methods 9, 805–807 (2012).
Liu, F., Song, Y. & Liu, D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 6, 1258–1266 (1999).
Khorsandi, S. E. et al. Minimally invasive and selective hydrodynamic gene therapy of liver segments in the pig and human. Cancer Gene Ther. 15, 225–230 (2008).
Mahiny, A. J. et al. In vivo genome editing using nuclease-encoding mRNA corrects SP-B deficiency. Nat. Biotechnol. 33, 584–586 (2015).
Wang, M. et al. Efficient delivery of genome-editing proteins using bioreducible lipid nanoparticles. Proc. Natl Acad. Sci. USA 113, 2868–2873 (2016).
Wang, H. et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918 (2013).
Yang, L. et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science 350, 1101–1104 (2015).
Liang, P. et al. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell 6, 363–372 (2015)
Baltimore, B. D. et al. A prudent path forward for genomic engineering and germline gene modification. Science 348, 36–38 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01543152 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT00842634 (2016).
Tebas, P. et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N. Engl. J. Med. 370, 901–910 (2014). This study tests the safety and feasibility of ZFNs targeting CCR5 in patients.
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02225665 (2015).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02500849 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02695160 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02702115 (2016).
Xue, W. et al. CRISPR-mediated direct mutation of cancer genes in the mouse liver. Nature 514, 380–385 (2014).
Mueller, C. et al. Sustained miRNA-mediated knockdown of mutant AAT with simultaneous augmentation of wild-type AAT has minimal effect on global liver miRNA profiles. Mol. Ther. 20, 590–600 (2012).
Chapman, J. R., Taylor, M. R. & Boulton, S. J. Playing the end game: DNA double-strand break repair pathway choice. Mol. Cell 47, 497–510 (2012).
Wu, W. H. et al. CRISPR repair reveals causative mutation in a preclinical model of retinitis pigmentosa. Mol. Ther. 24, 1388–1394 (2016).
Miller, D. G., Petek, L. M. & Russell, D. W. Human gene targeting by adeno-associated virus vectors is enhanced by DNA double-strand breaks. Mol. Cell. Biol. 23, 3550–3557 (2003).
Koudelka, K. J., Pitek, A. S., Manchester, M. & Steinmetz, N. F. Virus-based nanoparticles as versatile nanomachines. Annu. Rev. Virol. 2, 379–401 (2015).
Heyer, W. D., Ehmsen, K. T. & Liu, J. Regulation of homologous recombination in eukaryotes. Annu. Rev. Genet. 44, 113–139 (2010).
Moehle, E. A. et al. Targeted gene addition into a specified location in the human genome using designed zinc finger nucleases. Proc. Natl Acad. Sci. USA 104, 3055–3060 (2007).
Chen, F. et al. High-frequency genome editing using ssDNA oligonucleotides with zinc-finger nucleases. Nat. Methods 8, 753–755 (2011).
Maruyama, T. et al. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat. Biotechnol. 33, 538–542 (2015).
Chu, V. T. et al. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat. Biotechnol. 33, 543–548 (2015).
High, K. H., Nathwani, A., Spencer, T. & Lillicrap, D. Current status of haemophilia gene therapy. Haemophilia 20 (Suppl. 4), 43–49 (2014).
Doerfler, P. A. et al. Targeted approaches to induce immune tolerance for Pompe disease therapy. Mol. Ther. Methods Clin. Dev. 3, 15053 (2016).
Gaj, T., Epstein, B. E. & Schaffer, D. V. Genome engineering using adeno-associated virus: basic and clinical research applications. Mol. Ther. 24, 458–464 (2016).
Hacein-Bey-Abina, S. et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy. Science 302, 415–419 (2003).
Cavazzana-Calvo, M. et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 288, 669–672 (2000).
Chen, X., Gonçalves, M. A. et al. Engineered viruses as genome editing devices. Mol. Ther. 3, 447–457 (2016).
Waehler, R., Russell, S. J. & Curiel, D. T. Engineering targeted viral vectors for gene therapy. Nat. Rev. Genet. 8, 573–587 (2007).
Zincarelli, C., Soltys, S., Rengo, G. & Rabinowitz, J. E. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 302, 1073–1080 (2008).
Bouard, D., Alazard-Dany, D., Cosset, F. L., et al. Viral vectors: from virology to transgene expression. Br. J. Pharmacol. 157, 153–165 (2000).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT03041324 (2017).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01252641 (2015).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01079325 (2016).
US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT00748501 (2012).
Acknowledgements
H.Y., K.J.K. and D.G.A. acknowledge funding from the Koch Institute Marble Center for Cancer Nanomedicine and the Cancer Center Support (core) Grant P30-CA14051. H.Y. is supported by Skoltech Center. The authors apologize to those authors whose work was not cited directly owing to space limitations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
D.G.A. and H.Y. have applied for patents relating to delivery technologies for genome editing. D.G.A. is a scientific co-founder of CRISPR Therapeutics.
Related links
Glossary
- RNA interference
-
(RNAi). Process by which one strand of double-stranded RNA binds to complementary mRNA and degrades or regulates the mRNA via an enzymatic process, usually resulting in a decrease in the expression of a desired protein.
- Antisense oligonucleotides
-
(ASOs). Short single-stranded DNA or RNA sequences that bind to complementary mRNAs, inhibit translation and/or degrade the targeted mRNA, resulting in a decrease in the expression of a desired protein.
- Episomal
-
DNA that functions without integrating into the genome: for example, a delivered DNA plasmid.
- Protospacer adjacent motif
-
A short, typically 2–6 nucleotide-long region of DNA recognized by the Cas9 protein, located immediately next to the target region for the Cas9 nuclease.
- Tropism
-
The ability of a virus to specifically target particular cells or tissues.
- Allogeneic
-
From the same species but not genetically compatible; that is, will induce an immune response.
- Ribonucleoprotein
-
(RNP). Any biomacromolecule consisting of an RNA in complex with a protein.
- Hydrodynamic injection
-
A rapid, high-volume intravenous infusion.
- Monogenic
-
Under the control of a single gene.
Rights and permissions
About this article
Cite this article
Yin, H., Kauffman, K. & Anderson, D. Delivery technologies for genome editing. Nat Rev Drug Discov 16, 387–399 (2017). https://doi.org/10.1038/nrd.2016.280
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2016.280