Abstract
Peripheral arterial disease (PAD) is caused by occlusive atherosclerosis in a vascular bed other than the heart. The lower extremity is the most-common location for PAD. Critical limb ischaemia (CLI) is the most-severe clinical manifestation of PAD. Despite improvements in medical care and revascularization, patients with CLI continue to have a high risk of major amputation (below the knee or higher) and cardiovascular death. The primary goal of therapy in CLI is to achieve blood flow to the distal limb vessels with angioplasty or bypass surgery. However, many patients with CLI are unsuitable for revascularization, or the procedure is unsuccessful. Angiogenesis is the growth and proliferation of blood vessels from an existing vascular structure. In therapeutic angiogenesis, attempts are made to utilize blood vessel growth to augment perfusion. In this Review, data from phase II and III clinical trials of therapeutic angiogenesis in patients with PAD will be presented and discussed. Potential explanations for the limited success of therapeutic angiogenesis in humans will be viewed in the context of advances in our understanding of the complex mechanisms underlying angiogenesis and vascular remodelling. This Review will also cover how advances in systems biology, genetics, and gene therapy might still allow the development of new approaches to therapeutic angiogenesis and achieve the goal of restoring perfusion.
Key Points
-
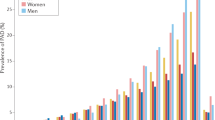
Peripheral arterial disease is a manifestation of systematic atherosclerosis that causes impaired blood flow to the leg; critical limb ischaemia is the most-severe form of peripheral arterial disease
-
Angiogenesis is the growth of blood vessels from existing vascular structures, whereas 'therapeutic angiogenesis' is a strategy that seeks to promote blood vessel growth to improve tissue perfusion
-
Vascular remodelling that is sufficient to augment tissue perfusion is likely to involve a combination of the processes of angiogenesis, arteriogenesis, and vasculogenesis
-
Although a series of randomized, double-blind, placebo-controlled trials in therapeutic angiogenesis have been completed, the degree of success in these studies has been limited
-
Future research directions in therapeutic angiogenesis and gene therapy include the identification of new vectors to improve gene transfer, as well as new genes designed to improve tissue perfusion
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Belch, J. J. et al. Critical issues in peripheral arterial disease detection and management: a call to action. Arch. Intern. Med. 163, 884–892 (2003).
Norgren, L. et al. Inter-society consensus for the management of peripheral arterial disease (TASCII). J. Vasc. Surg. 45 (Suppl. S), S5–S67 (2007).
Roger, V. L. et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 123, e18–e209 (2011).
Beckman, J. A., Creager, M. A. & Libby, P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 287, 2570–2581 (2002).
Dormandy, J., Heeck, L. & Vig, S. The fate of patients with critical leg ischemia. Semin. Vasc. Surg. 12, 142–147 (1999).
Hankey, G. J., Norman, P. E. & Eikelboom, J. W. Medical treatment of peripheral arterial disease. JAMA 295, 547–553 (2006).
Hiatt, W. R., Hoag, S. & Hamman, R. F. Effect of diagnostic criteria on the prevalence of peripheral arterial disease. The San Luis Valley Diabetes Study. Circulation 91, 1472–1479 (1995).
Hiatt, W. R. Medical treatment of peripheral arterial disease and claudication. N. Engl. J. Med. 344, 1608–1621 (2001).
Fontaine, R., Kim, M. & Kieny, R. Surgical treatment of peripheral circulation disorders [German]. Helv. Chir. Acta 21, 499–533 (1954).
Dormandy, J. A. & Rutherford, R. B. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J. Vasc. Surg. 31, S1–S296 (2000).
Brass, E. P. et al. Parenteral therapy with lipo-ecraprost, a lipid-based formulation of a PGE1 analog, does not alter six-month outcomes in patients with critical leg ischemia. J. Vasc. Surg. 43, 752–759 (2006).
Ouriel, K. Peripheral arterial disease. Lancet 358, 1257–1264 (2001).
Berceli, S. A. et al. Surgical and endovascular revision of infrainguinal vein bypass grafts: analysis of midterm outcomes from the PREVENT III trial. J. Vasc. Surg. 46, 1173–1179 (2007).
Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1, 27–31 (1995).
Simons, M. Angiogenesis: where do we stand now? Circulation 111, 1556–1566 (2005).
Losordo, D. W. & Dimmler, S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation 109, 2487–2491 (2004).
Asahara, T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967 (1997).
Ito, W. D. et al. Angiogenesis but not collateral growth is associated with ischemia after femoral artery occlusion. Am. J. Physiol. 273, H1255–H1265 (1997).
Robbins, J. L. et al. Relationship between leg muscle capillary density and peak hyperemic blood flow with endurance capacity in peripheral artery disease. J. Appl. Phys. 111, 81–86 (2011).
Askew, C. D. et al. Skeletal muscle phenotype is associated with exercise tolerance in patients with peripheral arterial disease. J. Vasc. Surg. 41, 802–807 (2005).
Mitchell, R. G. et al. Increased levels of apoptosis in gastrocnemius skeletal muscle in patients with peripheral arterial disease. Vasc. Med. 12, 285–290 (2007).
Duscha, B. D. et al. Angiogenesis in skeletal muscle precede improvements in peak oxygen uptake in peripheral artery disease patients. Arterioscler. Thromb. Vasc. Biol. 31, 2742–2748 (2011).
Gardner, A. W. & Killewich, L. A. Lack of functional benefits following infrainguinal bypass in peripheral arterial occlusive disease patients. Vasc. Med. 6, 9–14 (2001).
Schaper, W. & Buschmann, I. Arteriogenesis, the good and bad of it. Cardiovasc. Res. 43, 835–837 (1999).
Chalothorn, D. et al. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol. Genomics 30, 179–191 (2007).
de Muinck, E. D. & Simons, M. Re-evaluating therapeutic neovascularization. J. Mol. Cell. Cardiol. 36, 25–32 (2004).
Heil, M., Ziegelhoeffer, T., Mees, B. & Schaper, W. A different outlook on the role of bone marrow stem cells in vascular growth: bone marrow delivers software not hardware. Circ. Res. 94, 573–574 (2004).
Luttun, A. & Carmeliet, P. De novo vasculogenesis in the heart. Cardiovasc. Res. 58, 378–389 (2003).
Mac Gabhann, F., Qutub, A. A., Annex, B. H. & Popel, A. S. Systems biology of pro-angiogenic therapies targeting the VEGF system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2, 694–707 (2010).
Ferrara, N. Vascular endothelial growth factor. Arterioscler. Thromb. Vasc. Biol. 29, 789–791 (2009).
Vempati, P. Mac Gabhann, F. & Popel, A. S. Formation of VEGF isoform-specific spatial distributions governing angiogenesis: computational analysis. BMC Syst. Biol. 5, 59 (2011).
Qutub, A. A., Mac Gabhann, F., Karagiannis, E. D., Vempati, P. & Popel, A. S. Multiscale models of angiogenesis. IEEE Eng. Med. Biol. Mag. 28, 14–31 (2009).
Stefanini, M. O., Wu, F. T., Mac Gabhann, F. & Popel, A. S. A compartment model of VEGF distribution in blood, healthy and diseased tissues. BMC Syst. Biol. 2, 77 (2008).
Wu, F. T. et al. Computational kinetic model of VEGF trapping by soluble VEGF receptor-1: Effects of transendothelial and lymphatic macromolecular transport. Physiol. Genomics 38, 29–41 (2009).
Hazarika, S. et al. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ. Res. 101, 948–956 (2007).
Mac Gabhann, F. & Popel, A. S. Model of competitive binding of vascular endothelial growth factor and placental growth factor to VEGF receptors on endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 286, H153–H164 (2004).
Mac Gabhann, F., Annex, B. H. & Popel, A. S. Gene therapy from the perspective of systems biology. Curr. Opin. Mol. Ther. 12, 570–577 (2010).
Wu, F. T. et al. VEGF and soluble VEGF receptor-1 (sFlt-1) distributions in peripheral arterial disease: an in silico model. Am. J. Physiol. Heart Circ. Physiol. 298, H2174–H2191 (2010).
Folkman, J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285, 1182–1186 (1971).
Senger, D. R. et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219, 983–985 (1983).
Rajagopalan, S. et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation 108, 1933–1938 (2003).
de Vries, C. et al. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 255, 989–991 (1992).
Isner, J. M. et al. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet 348, 370–374 (1996).
Lederman, R. J. et al. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet 359, 2053–2058 (2002).
Simons, M. et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation 105, 788–793 (2002).
Grines, C. L. et al. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation 105, 1291–1297 (2002).
Kusumanto, Y. H. et al. Treatment with intramuscular vascular endothelial growth factor gene compared with placebo for patients with diabetes mellitus and critical limb ischemia: a double-blind randomized trial. Hum. Gene Ther. 17, 683–691 (2006).
Witzenbichler, B. et al. Intramuscular gene transfer of fibroblast growth factor-1 using improved pCOR plasmid design stimulates collateral formation in a rabbit ischemic hindlimb model. J. Molec. Med. 84, 491–502 (2006).
Comerota, A. J. et al. Naked plasmid DNA encoding fibroblast growth factor type 1 for the treatment of end-stage unreconstructible lower extremity ischemia: preliminary results of a phase I trial. J. Vasc. Surg. 35, 930–936 (2002).
Baumgartner, I. et al. Local gene transfer and expression following intramuscular administration of FGF-1 plasmid DNA in patients with critical limb ischemia. Mol. Ther. 17, 914–921 (2009).
Nikol, S. et al. Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia. Mol. Ther. 16, 972–978 (2008).
Belch, J. et al. Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet 377, 1929–1937 (2011).
Tashiro, K. et al. Deduced primary structure of rat hepatocyte growth factor and expression of the mRNA in rat tissues. Proc. Natl Acad. Sci. USA 87, 3200–3204 (1990).
Hayashi. S. et al. Potential role of hepatocyte growth factor, a novel angiogenic growth factor, in peripheral arterial disease: downregulation of HGF in response to hypoxia in vascular cells. Circulation 100 (19 Suppl.), II301–II308 (1999).
Powell, R. J. et al. Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation 118, 58–65 (2008).
Powell, R. J. et al. Safety and efficacy of patient specific intramuscular injection of HGF plasmid gene therapy on limb perfusion and wound healing in patients with ischemic lower extremity ulceration: results of the HGF-0205 trial. J. Vasc. Surg. 52, 1525–1530 (2010).
Shigematsu, H. et al. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther. 17, 1152–1161 (2010).
Creager, M. A. et al. Effect of hypoxia-inducible factor-1alpha gene therapy on walking performance in patients with intermittent claudication. Circulation 124, 1765–1773 (2011).
Chung, A. S., Lee, J. & Ferrara, N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat. Rev. Cancer 10, 505–514 (2010).
Ferrara, N. Vascular endothelial growth factor and age-related macular degeneration: from basic science to therapy. Nat. Med. 16, 1107–1111 (2010).
Tang, G. L. et al. The effect of gradual or acute arterial occlusion on skeletal muscle blood flow, arteriogenesis, and inflammation in rat hindlimb ischemia. J. Vasc. Surg. 41, 312–320 (2005).
Waters, R. E., Terjung, R. L., Peters, K. G. & Annex, B. H. Preclinical models of human peripheral arterial occlusive disease: implications for investigation of therapeutic agents. J. Appl. Physiol. 97, 773–780 (2004).
Dokun A. O. et al. A quantitative trait locus (LSq-1) on mouse chromosome 7 is linked to the absence of tissue loss after surgical hindlimb ischemia. Circulation 117, 1207–1215 (2008).
Allen, J. D. et al. Plasma nitrite response and arterial reactivity differentiate vascular health and performance. Nitric Oxide 20, 231–237 (2009).
Sun, L., Bai, Y. & Du, G. Endothelial dysfunction—an obstacle of therapeutic angiogenesis. Ageing Res. Rev. 8, 306–313 (2009).
Tuomisto, T. T. et al. HIF-VEGF-VEGFR-2, TNF-alpha and IGF pathways are upregulated in critical human skeletal muscle ischemia as studied with DNA array. Atherosclerosis 174, 111–120 (2004).
Nakamura, T. et al. Myocardial protection from ischemia/reperfusion injury by endogenous and exogenous HGF. J. Clin. Invest. 106, 1511–1519 (2000).
Yamamura, Y. et al. IGF-I differentially regulates Bcl-xL and Bax and confers myocardial protection in the rat heart. Am. J. Physiol. Heart Circ. Physiol. 280, H1191–H1200 (2001).
Helisch, A. et al. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler. Thromb. Vasc. Biol. 26, 520–526 (2006).
Reny, J. L. et al. The factor II G20210A gene polymorphism, but not factor V Arg506Gln, is associated with peripheral arterial disease: results of a case–control study. J. Thromb. Haemost. 2, 1334–1340 (2004).
Fontana, P. et al. P2Y12 H2 haplotype is associated with peripheral arterial disease: a case–control study. Circulation 108, 2971–2973 (2003).
Fowkes, F. G. et al. Fibrinogen genotype and risk of peripheral atherosclerosis. Lancet 339, 693–696 (1992).
Gudmundsson, G. et al. Localization of a gene for peripheral arterial occlusive disease to chromosome 1p31. Am. J. Hum. Genet. 70, 586–592 (2002).
Murabito, J. M. et al. Association between chromosome 9p21 variants and the ankle–brachial index identified by a meta-analysis of 21 genome-wide association studies. Circ. Cardiovasc. Genet. 5, 100–112 (2012).
Da Silva, A. et al. The Basle longitudinal study: report on the relation of initial glucose level to baseline ECG abnormalities, peripheral artery disease, and subsequent mortality. J. Chronic Dis. 32, 797–803 (1979).
Willigendael, E. M. et al. Influence of smoking on incidence and prevalence of peripheral arterial disease. J. Vasc. Surg. 40, 1158–1165 (2004).
Scholz, D. et al. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J. Mol. Cell. Cardiol. 34, 775–787 (2002).
Fukino, K., Sata, M., Seko, Y., Hirata, Y. & Nagai, R. Genetic background influences therapeutic effectiveness of VEGF. Biochem. Biophys. Res. Commun. 310, 143–147 (2003).
Rissanen, T. T. & Ylä-Herttuala, S. Current status of cardiovascular gene therapy. Mol. Ther. 15, 1233–1247 (2007).
Kotchey, N. M. et al. A potential role of distinctively delayed blood clearance of recombinant adeno-associated virus serotype 9 in robust cardiac transduction. Mol. Ther. 19, 1079–1089 (2011).
Hernandez, Y. J. et al. Latent adeno-associated virus infection elicits humoral but not cell-mediated immune responses in a nonhuman primate model. J. Virol. 73, 8549–8558 (1999).
Saqib, A. et al. Adeno-associated virus serotype 9-mediated overexpression of extracellular superoxide dismutase improves recovery from surgical hind-limb ischemia in BALB/c mice. J. Vasc. Surg. 54, 810–818 (2011).
Katwal, A. B. et al. Adeno-associated virus serotype 9 efficiently targets ischemic skeletal muscle following systemic delivery in a mouse model of peripheral arterial disease [abstract A14001]. Circulation 124, A14001 (2011).
Acknowledgements
The author's work is supported by grant 1R01HL101200 from the National Heart Lung and Blood Institute.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author has acted as a consultant for AnGes MG.
Rights and permissions
About this article
Cite this article
Annex, B. Therapeutic angiogenesis for critical limb ischaemia. Nat Rev Cardiol 10, 387–396 (2013). https://doi.org/10.1038/nrcardio.2013.70
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2013.70