Abstract
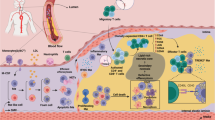
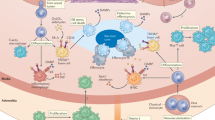
Chronic inflammation drives atherosclerosis, the leading cause of cardiovascular disease. Over the past two decades, data have emerged showing that immune cells are involved in the pathogenesis of atherosclerotic plaques. The accumulation and continued recruitment of leukocytes are associated with the development of 'vulnerable' plaques. These plaques are prone to rupture, leading to thrombosis, myocardial infarction or stroke, all of which are frequent causes of death. Plaque macrophages account for the majority of leukocytes in plaques, and are believed to differentiate from monocytes recruited from circulating blood. However, monocytes represent a heterogenous circulating population of cells. Experiments are needed to address whether monocyte recruitment to plaques and effector functions, such as the formation of foam cells, the production of nitric oxide and reactive oxygen species, and proteolysis are critical for the development and rupture of plaques, and thus for the pathophysiology of atherosclerosis, as well as elucidate the precise mechanisms involved.
Key Points
-
Atherosclerosis has long been associated with chronic inflammation
-
The accumulation of macrophages correlates with atherosclerotic plaque progression and plaque rupture
-
Monocytes accumulate at sites of inflammation and have been reported to enter atherosclerotic plaques
-
Macrophages and foam cell are present in plaques and might derive from monocytes or from resident macrophages
-
Monocytes represent a heterogeneous population of cells with differences in phenotype, function and locomotion
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ross, R. Atherosclerosis—an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Gerrity, R. G., Naito, H. K., Richardson, M. & Schwartz, C. J. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am. J. Pathol. 95, 775–792 (1979).
Hansson, G. K. & Libby, P. The immune response in atherosclerosis: a double-edged sword. Nat. Rev. Immunol. 6, 508–519 (2006).
Galkina, E. & Ley, K. Leukocyte influx in atherosclerosis. Curr. Drug Targets 8, 1239–1248 (2007).
Weber, C., Zernecke, A. & Libby, P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat. Rev. Immunol. 8, 802–815 (2008).
Heeneman, S., Lutgens, E., Schapira, K. B., Daemen, M. J. & Biessen, E. A. Control of atherosclerotic plaque vulnerability: insights from transgenic mice. Front. Biosci. 13, 6289–6313 (2008).
Mallat, Z., Taleb, S., Ait-Oufella, H. & Tedgui, A. The role of adaptive T cell immunity in atherosclerosis. J. Lipid Res. 50 (Suppl.), S364–S369 (2009).
Auffray, C., Sieweke, M. H. & Geissmann, F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 27, 669–692 (2009).
Swirski, F. K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009).
Geissmann, F., Jung, S. & Littman, D. R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 (2003).
Geissmann, F. et al. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol. Cell Biol. 86, 398–408 (2008).
Tacke, F. & Randolph, G. J. Migratory fate and differentiation of blood monocyte subsets. Immunobiology 211, 609–618 (2006).
Varol, C., Yona, S. & Jung, S. Origins and tissue-context-dependent fates of blood monocytes. Immunol. Cell Biol. 87, 30–38 (2009).
Imhof, B. A. & Aurrand-Lions, M. Adhesion mechanisms regulating the migration of monocytes. Nat. Rev. Immunol. 4, 432–444 (2004).
Ley, K., Laudanna, C., Cybulsky, M. I. & Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 (2007).
Greaves, D. R. & Gordon, S. The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J. Lipid Res. 50 (Suppl.), S282–S286 (2009).
Galkina, E. & Ley, K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu. Rev. Immunol. 27, 165–197 (2009).
Hahn, C. & Schwartz, M. A. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 10, 53–62 (2009).
Bjornheden, T., Levin, M., Evaldsson, M. & Wiklund, O. Evidence of hypoxic areas within the arterial wall in vivo. Arterioscler. Thromb. Vasc. Biol. 19, 870–876 (1999).
Virmani, R. et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler. Thromb. Vasc. Biol. 25, 2054–2061 (2005).
Moulton, K. S. et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc. Natl Acad. Sci. USA 100, 4736–4741 (2003).
Newby, A. C. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol. Rev. 85, 1–31 (2005).
Fuster, V., Moreno, P. R., Fayad, Z. A., Corti, R. & Badimon, J. J. Atherothrombosis and high-risk plaque: part I: evolving concepts. J. Am. Coll. Cardiol. 46, 937–954 (2005).
Primatesta, P. et al. Cardiovascular surveys: manual of operations. Eur. J. Cardiovasc. Prev. Rehabil. 14 (Suppl. 3), S43–S61 (2007).
Shah, P. K. Molecular mechanisms of plaque instability. Curr. Opin. Lipidol. 18, 492–499 (2007).
Llodra, J. et al. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc. Natl Acad. Sci. USA 101, 11779–11784 (2004).
Feig, J. E., Quick, J. S. & Fisher, E. A. The role of a murine transplantation model of atherosclerosis regression in drug discovery. Curr. Opin. Investig. Drugs 10, 232–238 (2009).
Randolph, G. J. Emigration of monocyte-derived cells to lymph nodes during resolution of inflammation and its failure in atherosclerosis. Curr. Opin. Lipidol. 19, 462–468 (2008).
Cybulsky, M. I., Won, D. & Haidari, M. Leukocyte recruitment to atherosclerotic lesions. Can. J. Cardiol. 20 (Suppl. B), 24B–28B (2004).
Swirski, F. K., Weissleder, R. & Pittet, M. J. Heterogeneous in vivo behavior of monocyte subsets in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 29, 1424–1432 (2009).
Trogan, E. et al. Laser capture microdissection analysis of gene expression in macrophages from atherosclerotic lesions of apolipoprotein E-deficient mice. Proc. Natl Acad. Sci. USA 99, 2234–2239 (2002).
Palframan, R. T. et al. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 194, 1361–1373 (2001).
Qu, C. et al. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J. Exp. Med. 200, 1231–1241 (2004).
Randolph, G. J., Beaulieu, S., Lebecque, S., Steinman, R. M. & Muller, W. A. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science 282, 480–483 (1998).
Taylor, P. R. & Gordon, S. Monocyte heterogeneity and innate immunity. Immunity 19, 2–4 (2003).
Auffray, C. et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 3 17, 666–670 (2007).
Audoy-Remus, J. et al. Rod-shaped monocytes patrol the brain vasculature and give rise to perivascular macrophages under the influence of proinflammatory cytokines and angiopoietin-2. J. Neurosci. 28, 10187–10199 (2008).
Gordon, S. & Taylor, P. R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 (2005).
Napoli, C. et al. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J. Clin. Invest. 100, 2680–2690 (1997).
Ylitalo, R., Oksala, O., Yla-Herttuala, S. & Ylitalo, P. Effects of clodronate (dichloromethylene bisphosphonate) on the development of experimental atherosclerosis in rabbits. J. Lab. Clin. Med. 123, 769–776 (1994).
Stoneman, V. et al. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ. Res. 100, 884–893 (2007).
Plump, A. S. & Breslow, J. L. Apolipoprotein E and the apolipoprotein E-deficient mouse. Annu. Rev. Nutr. 15, 495–518 (1995).
Combadiere, C. et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 11 7, 1649–1657 (2008).
Zhang, S. H., Reddick, R. L., Piedrahita, J. A. & Maeda, N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 258, 468–471 (1992).
Zernecke, A. et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ. Res. 102, 209–217 (2008).
Swirski, F. K. et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117, 195–205 (2007).
Tacke, F. et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117, 185–194 (2007).
Dunon, D., Piali, L. & Imhof, B. A. To stick or not to stick: the new leukocyte homing paradigm. Curr. Opin. Cell Biol. 8, 714–723 (1996).
Springer, T. A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 57, 827–872 (1995).
Zernecke, A., Shagdarsuren, E. & Weber, C. Chemokines in atherosclerosis: an update. Arterioscler. Thromb. Vasc. Biol. 28, 1897–1908 (2008).
Charo, I. F. & Taubman, M. B. Chemokines in the pathogenesis of vascular disease. Circ. Res. 95, 858–866 (2004).
Saederup, N., Chan, L., Lira, S. A. & Charo, I. F. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2−/− mice: evidence for independent chemokine functions in atherogenesis. Circulation 117, 1642–1648 (2008).
Lesnik, P., Haskell, C. A. & Charo, I. F. Decreased atherosclerosis in CX3CR1−/− mice reveals a role for fractalkine in atherogenesis. J. Clin. Invest. 111, 333–340 (2003).
Ancuta, P. et al. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J. Exp. Med. 197, 1701–1707 (2003).
Landsman, L. et al. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood 11 3, 963–972 (2009).
Serbina, N. V. & Pamer, E. G., Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7, 311–317 (2006).
Woollard, K. J. & Chin-Dusting, J. Therapeutic targeting of p-selectin in atherosclerosis. Inflamm. Allergy Drug Targets. 6, 69–74 (2007).
Girard, J. P. & Springer, T. A. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol. Today 16, 449–457 (1995).
Johnson-Tidey, R. R., McGregor, J. L., Taylor, P. R. & Poston, R. N. Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques. Coexpression with intercellular adhesion molecule-1. Am. J. Pathol. 144, 952–961 (1994).
Johnson, R. C. et al. Absence of P-selectin delays fatty streak formation in mice. J. Clin. Invest. 99, 1037–1043 (1997).
Katayama, Y. et al. PSGL-1 participates in E-selectin-mediated progenitor homing to bone marrow: evidence for cooperation between E-selectin ligands and alpha4 integrin. Blood 1 02, 2060–2067 (2003).
Collins, R. G. et al. P-Selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J. Exp. Med. 191, 189–194 (2000).
Dong, Z. M. et al. The combined role of P- and E-selectins in atherosclerosis. J. Clin. Invest. 102, 145–152 (1998).
Woollard, K. J. Soluble bio-markers in vascular disease: much more than gauges of disease? Clin. Exp. Pharmacol. Physiol. 32, 233–240 (2005).
Woollard, K. J. et al. Pathophysiological levels of soluble P-selectin mediate adhesion of leukocytes to the endothelium through Mac-1 activation. Circ. Res. 103, 1128–1138 (2008).
Woollard, K. J. et al. Raised plasma soluble P-selectin in peripheral arterial occlusive disease enhances leukocyte adhesion. Circ. Res. 98, 149–156 (2006).
Kisucka, J. et al. Elevated levels of soluble P-selectin in mice alter blood–brain barrier function, exacerbate stroke, and promote atherosclerosis. Blood 1 13, 6015–6022 (2009).
An, G. et al. P-selectin glycoprotein ligand-1 is highly expressed on Ly-6Chi monocytes and a major determinant for Ly-6Chi monocyte recruitment to sites of atherosclerosis in mice. Circulation 117, 3227–3237 (2008).
Grage-Griebenow, E., Flad, H. D. & Ernst, M. Heterogeneity of human peripheral blood monocyte subsets. J. Leukoc. Biol. 69, 11–20 (2001).
Galkina, E. & Ley, K. Vascular adhesion molecules in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 27, 2292–2301 (2007).
Patel, S. S., Thiagarajan, R., Willerson, J. T. & Yeh, E. T. Inhibition of alpha4 integrin and ICAM-1 markedly attenuate macrophage homing to atherosclerotic plaques in ApoE-deficient mice. Circulation 97, 75–81 (1998).
Nageh, M. F. et al. Deficiency of inflammatory cell adhesion molecules protects against atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 17, 1517–1520 (1997).
Cybulsky, M. I. et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Invest. 107, 1255–1262 (2001).
Chatzizisis, Y. S. et al. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J. Am. Coll. Cardiol. 49, 2379–2393 (2007).
Eriksson, E. E., Werr, J., Guo, Y., Thoren, P. & Lindbom, L. Direct observations in vivo on the role of endothelial selectins and alpha(4) integrin in cytokine-induced leukocyte–endothelium interactions in the mouse aorta. Circ. Res. 86, 526–533 (2000).
Alvarez, A. et al. Direct evidence of leukocyte adhesion in arterioles by angiotensin II. Blood 104, 402–408 (2004).
Caputo, K. E., Lee, D., King, M. R. & Hammer, D. A. Adhesive dynamics simulations of the shear threshold effect for leukocytes. Biophys. J. 92, 787–797 (2007).
Chauhan, A. K. et al. ADAMTS13: a new link between thrombosis and inflammation. J. Exp. Med. 205, 2065–2074 (2008).
Bernardo, A. et al. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J. Thromb. Haemost. 3, 562–570 (2005).
Soehnlein, O., Lindbom, L. & Weber, C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood 114, 4613–4623 (2009).
Newby, A. C. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler. Thromb. Vasc. Biol. 28, 2108–2114 (2008).
Hazen, S. L. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J. Biol. Chem. 283, 15527–15531 (2008).
Febbraio, M. et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Invest. 105, 1049–1056 (2000).
Kuchibhotla, S. et al. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc. Res. 78, 185–196 (2008).
Moore, K. J. et al. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J. Clin. Invest. 115, 2192–2201 (2005).
Park, Y. M., Febbraio, M. & Silverstein, R. L. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J. Clin. Invest. 119, 136–145 (2009).
Moreno, P. R., Purushothaman, K. R., Sirol, M., Levy, A. P. & Fuster, V. Neovascularization in human atherosclerosis. Circulation 113, 2245–2252 (2006).
Barger, A. C., Beeuwkes, R., 3rd, Lainey, L. L. & Silverman, K. J. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N. Engl. J. Med. 310, 175–177 (1984).
Celletti, F. L. et al. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat. Med. 7, 425–429 (2001).
Swirski, F. K. et al. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc. Natl Acad. Sci. USA 103, 10340–10345 (2006).
Tabas, I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler. Thromb. Vasc. Biol. 25, 2255–2264 (2005).
Passlick, B., Flieger, D. & Ziegler-Heitbrock, H. W. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 74, 2527–2534 (1989).
Strauss-Ayali, D., Conrad, S. M. & Mosser, D. M. Monocyte subpopulations and their differentiation patterns during infection. J. Leukoc. Biol. 82, 244–252 (2007).
Weber, C. et al. Differential chemokine receptor expression and function in human monocyte subpopulations. J. Leukoc. Biol. 67, 699–704 (2000).
Salomon, R. N., Underwood, R., Doyle, M. V., Wang, A. & Libby, P. Increased apolipoprotein E and c-fms gene expression without elevated interleukin 1 or 6 mRNA levels indicates selective activation of macrophage functions in advanced human atheroma. Proc. Natl Acad. Sci. USA 89, 2814–2818 (1992).
Schlitt, A. et al. CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb. Haemost. 92, 419–424 (2004).
Rothe, G. et al. Peripheral blood mononuclear phagocyte subpopulations as cellular markers in hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 16, 1437–1447 (1996).
Tsujioka, H. et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J. Am. Coll. Cardiol. 54, 130–138 (2009).
Wildgruber, M. et al. Monocyte subset dynamics in human atherosclerosis can be profiled with magnetic nano-sensors. PLoS One 4, e5663 (2009).
Acknowledgements
K. J. Woollard is funded by a British Heart Foundation fellowship. Work in F. Geissmann's lab is supported by the Arthritis Research Campaign and the Medical Research Council (G0900867 Strategic Award).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Woollard, K., Geissmann, F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 7, 77–86 (2010). https://doi.org/10.1038/nrcardio.2009.228
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2009.228