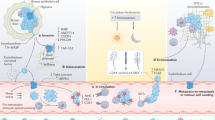
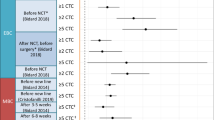
Abstract
During the past ten years, circulating tumour cells (CTCs) have received enormous attention as new biomarkers and the subject of basic research. Although CTCs are already used in numerous clinical trials, their clinical utility is still under investigation. Many issues regarding the detection and characterization of CTCs remain unknown. In this Opinion article, we propose a conceptual framework of CTC assays and point out current challenges of CTC research, which might structure this dynamic field of translational cancer research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Zhang, L. et al. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin. Cancer Res. 18, 5701–5710 (2012).
Scher, H. I. et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 10, 233–239 (2009).
Hou, J. M. et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 30, 525–532 (2012).
Krebs, M. G. et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J. Clin. Oncol. 29, 1556–1563 (2011).
Aggarwal, C. et al. Relationship among circulating tumor cells, CEA and overall survival in patients with metastatic colorectal cancer. Ann. Oncol. 24, 420–428 (2013).
Deneve, E. et al. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clin. Chem. 59, 1384–1392 (2013).
Bidard, F. C. et al. Clinical application of circulating tumor cells in breast cancer: overview of the current interventional trials. Cancer Metastasis Rev. 32, 179–188 (2013).
Pantel, K. & Alix-Panabieres, C. Real-time liquid biopsy in cancer patients: fact or fiction? Cancer Res. 73, 6384–6388 (2013).
Zippelius, A. et al. Limitations of reverse-transcriptase polymerase chain reaction analyses for detection of micrometastatic epithelial cancer cells in bone marrow. J. Clin. Oncol. 15, 2701–2708 (1997).
Pantel, K. & Alix-Panabieres, C. The clinical significance of circulating tumor cells. Nature Clin. Pract. Oncol. 4, 62–63 (2007).
Pantel, K., Alix-Panabieres, C. & Riethdorf, S. Cancer micrometastases. Nature Rev. Clin. Oncol. 6, 339–351 (2009).
Pantel, K. & Alix-Panabieres, C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol. Med. 16, 398–406 (2010).
Pantel, K. et al. Circulating epithelial cells in patients with benign colon diseases. Clin. Chem. 58, 936–940 (2012).
Rao, C. G. et al. Expression of epithelial cell adhesion molecule in carcinoma cells present in blood and primary and metastatic tumors. Int. J. Oncol. 27, 49–57 (2005).
Bednarz, N. et al. BRCA1 loss preexisting in small subpopulations of prostate cancer is associated with advanced disease and metastatic spread to lymph nodes and peripheral blood. Clin. Cancer Res. 16, 3340–3348 (2010).
Lee, J. M., Dedhar, S., Kalluri, R. & Thompson, E. W. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J. Cell Biol. 172, 973–981 (2006).
Yu, M. et al. RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature 487, 510–513 (2012).
Yu, M. et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584 (2013).
Yokobori, T. et al. Plastin3 is a novel marker for circulating tumor cells undergoing the epithelial-mesenchymal transition and is associated with colorectal cancer prognosis. Cancer Res. 73, 2059–2069 (2013).
Riethdorf, S. et al. Detection and HER2 Expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant geparquattro trial. Clin. Cancer Res. 16, 2634–2645 (2010).
Ignatiadis, M. et al. HER2-positive circulating tumor cells in breast cancer. PLoS ONE 6, e15624 (2011).
Miyamoto, D. T. et al. Androgen receptor signaling in circulating tumor cells as a marker of hormonally responsive prostate cancer. Cancer Discov. 2, 995–1003 (2012).
Markou, A., Strati, A., Malamos, N., Georgoulias, V. & Lianidou, E. S. Molecular characterization of circulating tumor cells in breast cancer by a liquid bead array hybridization assay. Clin. Chem. 57, 421–430 (2011).
Kufer, P. et al. Heterogeneous expression of MAGE-A genes in occult disseminated tumor cells: a novel multimarker reverse transcription-polymerase chain reaction for diagnosis of micrometastatic disease. Cancer Res. 62, 251–261 (2002).
Riethdorf, S. et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin. Cancer Res. 13, 920–928 (2007).
Coumans, F. A., van Dalum, G., Beck, M. & Terstappen, L. W. Filter characteristics influencing circulating tumor cell enrichment from whole blood. PLoS ONE 8, e61770 (2013).
Lustberg, M. B. et al. Heterogeneous atypical cell populations are present in blood of metastatic breast cancer patients. Breast Cancer Res. 16, R23 (2014).
Yang, L. et al. Optimization of an enrichment process for circulating tumor cells from the blood of head and neck cancer patients through depletion of normal cells. Biotechnol. Bioeng. 102, 521–534 (2009).
Shibata, K., Mori, M., Kitano, S. & Akiyoshi, T. Detection of ras gene mutations in peripheral blood of carcinoma patients using CD45 immunomagnetic separation and nested mutant allele specific amplification. Int. J. Oncol. 12, 1333–1338 (1998).
Ramirez, J. M. et al. Prognostic relevance of viable circulating tumor cells detected by EPISPOT in metastatic breast cancer patients. Clin. Chem. 60, 214–221 (2014).
Lin, H. K. et al. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin. Cancer Res. 16, 5011–5018 (2010).
Hou, H. W. et al. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci. Rep. 3, 1259 (2013).
Sollier, E. et al. Size-selective collection of circulating tumor cells using Vortex technology. Lab. Chip 14, 63–77 (2014).
Nedosekin, D. A. et al. Photoacoustic and photothermal detection of circulating tumor cells, bacteria and nanoparticles in cerebrospinal fluid in vivo and ex vivo. J. Biophoton. 6, 523–533 (2013).
Friedlander, T. W. et al. Detection and characterization of invasive circulating tumor cells (ictcs) derived from men with metastatic castration resistant prostate cancer (mCRPC). Int. J. Cancer 134, 2284–2293 (2013).
Baccelli, I. et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nature Biotech. 31, 539–544 (2013).
Hodgkinson, C. L. et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nature Med. http://dx.doi.org/10.1038/nm.3600 (2014).
Rhim, A. D. et al. EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–361 (2012).
Husemann, Y. et al. Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68 (2008).
Effenberger, K. E. et al. Disseminated tumor cells in pancreatic cancer-an independent prognosticator of disease progression and survival. Int. J. Cancer 131, E475–E483 (2012).
Nagrath, S. et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450, 1235–1239 (2007).
Stott, S. L. et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl Acad. Sci. USA 107, 18392–18397 (2010).
Ozkumur, E. et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci Transl. Med. 5, 179ra47 (2013).
Stott, S. L. et al. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci Transl. Med. 2, 25ra23 (2010).
Saucedo-Zeni, N. et al. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. Int. J. Oncol. 41, 1241–1250 (2012).
Eifler, R. L. et al. Enrichment of circulating tumor cells from a large blood volume using leukapheresis and elutriation: proof of concept. Cytometry B Clin. Cytom. 80, 100–111 (2011).
Fischer, J. C. et al. Diagnostic leukapheresis enables reliable detection of circulating tumor cells of nonmetastatic cancer patients. Proc. Natl Acad. Sci. USA 110, 16580–16585 (2013).
Hou, J. M. et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am. J. Pathol. 178, 989–996 (2011).
Meng, S. et al. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. 10, 8152–8162 (2004).
Bellahcene, A. et al. Transcriptome analysis reveals an osteoblast-like phenotype for human osteotropic breast cancer cells. Breast Cancer Res. Treat. 101, 135–148 (2007).
Garcia, T. et al. A convenient clinically relevant model of human breast cancer bone metastasis. Clin. Exp. Metastasis 25, 33–42 (2008).
Le Gall, C. et al. A cathepsin K inhibitor reduces breast cancer induced osteolysis and skeletal tumor burden. Cancer Res. 67, 9894–9902 (2007).
Bos, P. D. et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009 (2009).
Wan, L., Pantel, K. & Kang, Y. Tumor metastasis: moving new biological insights into the clinic. Nature Med. 19, 1450–1464 (2013).
Zhang, L. et al. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci Transl. Med. 5, 180ra48 (2013).
Labelle, M., Begum, S. & Hynes, R. O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20, 576–590 (2011).
Kang, Y. & Pantel, K. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell 23, 573–581 (2013).
Ocana, O. H. et al. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell 22, 709–724 (2012).
Tsai, J. H., Donaher, J. L., Murphy, D. A., Chau, S. & Yang, J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 22, 725–736 (2012).
Tsuji, T. et al. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res. 68, 10377–10386 (2008).
Tam, W. L. & Weinberg, R. A. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nature Med. 19, 1438–1449 (2013).
Punnoose, E. A. et al. Molecular biomarker analyses using circulating tumor cells. PLoS ONE 5, e12517 (2010).
Giordano, A. et al. Epithelial-mesenchymal transition and stem cell markers in patients with HER2-positive metastatic breast cancer. Mol. Cancer Ther. 11, 2526–2534 (2012).
Alix-Panabieres, C. et al. Detection and characterization of putative metastatic precursor cells in cancer patients. Clin. Chem. 53, 537–539 (2007).
Smerage, J. B. et al. Monitoring apoptosis and Bcl-2 on circulating tumor cells in patients with metastatic breast cancer. Mol. Oncol. 7, 680–692 (2013).
Muller, A. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 (2001).
Fusi, A. et al. Expression of chemokine receptors on circulating tumor cells in patients with solid tumors. J. Transl. Med. 10, 52 (2012).
Kaifi, J. T. et al. Tumor-cell homing to lymph nodes and bone marrow and CXCR4 expression in esophageal cancer. J. Natl Cancer Inst. 97, 1840–1847 (2005).
Shiozawa, Y. et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J. Clin. Invest. 121, 1298–1312 (2011).
Brugger, W. et al. Mobilization of tumor cells and hematopoietic progenitor cells into peripheral blood of patients with solid tumors. Blood 83, 636–640 (1994).
Joseph, J. et al. Disseminated prostate cancer cells can instruct hematopoietic stem and progenitor cells to regulate bone phenotype. Mol. Cancer Res. 10, 282–292 (2012).
Pantel, K. & Brakenhoff, R. H. Dissecting the metastatic cascade. Nature Rev. Cancer 4, 448–456 (2004).
Aguirre-Ghiso, J. A. Models, mechanisms and clinical evidence for cancer dormancy. Nature Rev. Cancer 7, 834–846 (2007).
Wicha, M. S. & Hayes, D. F. Circulating tumor cells: not all detected cells are bad and not all bad cells are detected. J. Clin. Oncol. 29, 1508–1511 (2011).
Krebs, M. G. et al. Molecular analysis of circulating tumour cells-biology and biomarkers. Nat. Rev. Clin. Oncol. 11, 129–144 (2014).
Wels, J., Kaplan, R. N., Rafii, S. & Lyden, D. Migratory neighbors and distant invaders: tumor-associated niche cells. Genes Dev. 22, 559–574 (2008).
Psaila, B. & Lyden, D. The metastatic niche: adapting the foreign soil. Nature Rev. Cancer 9, 285–293 (2009).
Heitzer, E. et al. Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer Res. 73, 2965–2975 (2013).
Lohr, J. G. et al. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nature Biotech. 32, 479–484 (2014).
Klein, C. A. Parallel progression of primary tumours and metastases. Nat. Rev. Cancer 9, 302–312 (2009).
Maheswaran, S. et al. Detection of mutations in EGFR in circulating lung-cancer cells. N. Engl. J. Med. 359, 366–377 (2008).
Gasch, C. et al. Heterogeneity of epidermal growth factor receptor status and mutations of KRAS/PIK3CA in circulating tumor cells of patients with colorectal cancer. Clin. Chem. 59, 252–260 (2013).
Mostert, B. et al. KRAS and BRAF mutation status in circulating colorectal tumor cells and their correlation with primary and metastatic tumor tissue. Int. J. Cancer 133, 130–141 (2013).
Fehm, T. et al. HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res. Treat. 124, 403–412 (2010).
Babayan, A. et al. Heterogeneity of estrogen receptor expression in circulating tumor cells from metastatic breast cancer patients. PLoS ONE 8, e75038 (2013).
Shaffer, D. R. et al. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin. Cancer Res. 13, 2023–2029 (2007).
Leversha, M. A. et al. Fluorescence in situ hybridization analysis of circulating tumor cells in metastatic prostate cancer. Clin. Cancer Res. 15, 2091–2097 (2009).
Jiang, Y., Palma, J. F., Agus, D. B., Wang, Y. & Gross, M. E. Detection of androgen receptor mutations in circulating tumor cells in castration-resistant prostate cancer. Clin. Chem. 56, 1492–1495 (2010).
Sridhar, S. S. et al. Castration-resistant prostate cancer: from new pathophysiology to new treatment. Eur. Urol. 65, 289–299 (2014).
Thorban, S. et al. Immunocytochemical detection of disseminated tumor cells in the bone marrow of patients with esophageal carcinoma. J. Natl Cancer Inst. 88, 1222–1227 (1996).
Pantel, K. & Alix-Panabieres, C. The potential of circulating tumor cells as a liquid biopsy to guide therapy in prostate cancer. Cancer Discov. 2, 974–975 (2012).
Smerage, J. B. et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J. Clin. Oncol. http://dx.doi.org/10.1200/JCO.2014.56.2561 (2014).
Scher, H. I., Morris, M. J., Larson, S. & Heller, G. Validation and clinical utility of prostate cancer biomarkers. Nat. Rev. Clin. Oncol. 10, 225–234 (2013).
Sieuwerts, A. M. et al. mRNA and microRNA expression profiles in circulating tumor cells and primary tumors of metastatic breast cancer patients. Clin. Cancer Res. 17, 3600–3618 (2011).
Powell, A. A. et al. Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS ONE 7, e33788 (2012).
Dawson, S. J. et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N. Engl. J. Med. 368, 1199–1209 (2013).
Diaz, L. A. Jr & Bardelli, A. Liquid biopsies: genotyping circulating tumor DNA. J. Clin. Oncol. 32, 579–586 (2014).
Kidess, E. & Jeffrey, S. S. Circulating tumor cells versus tumor-derived cell-free DNA: rivals or partners in cancer care in the era of single-cell analysis? Genome Med. 5, 70 (2013).
Bidard, F. C. et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 15, 406–414 (2014).
Murtaza, M. et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 497, 108–112 (2013).
Acknowledgements
C.A.-P. is supported by Grant “INCa-DGOS-Inserm 6045”, Institut National du Cancer (INCa) national grants; K.P. is supported by European Research Council Investigator Grant “DISSECT” (no. 269081); K.P. and C.A.-P. are both supported by the ERA-NET on Translational Cancer Research (TRANSCAN) grant “CTC-SCAN”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
C.A.P. received honoraria from Sanofi and grants from Janssen and Roche; K.P. received honoraria from Janssen, Alere and Gilupi.
Related links
Glossary
- BEAMing PCR
-
A combination of emulsion digital PCR and flow cytometry: beads, emulsions, amplification and magnetics (BEAMing) are combined to achieve the necessary level of sensitivity.
- Cancer stem cells
-
Cancer cells with self-renewing capacity and the ability to create or sustain a tumour cell population.
- Castration-resistant prostate cancer
-
(CRPC). Prostate cancer that no longer responds to androgen deprivation therapy.
- CellSearch® system
-
US Food and Drug Administration (FDA)-cleared technology that allows a sensitive positive capture of CTCs by antibodies against epithelial cell adhesion molecule (EPCAM) coated with ferrofluids: tumour cells are identified by positive immunostaining for antibodies against cytokeratins (CK8, CK18 and CK19), negative immunostaining for the common leukocyte antigen CD45 to exclude leukocytes, and positive DAPI staining as a measure of nuclear integrity.
- Clinical utility
-
The capacity to diagnose and to facilitate a decision to adopt or reject a therapeutic action: the risks and benefits result from test use.
- Clinical validity
-
The predictive value of a test for a given clinical outcome (for example, in cancer, a primary tumour or metastasis will develop in a patient with a positive test): a test identifies the clinical status of a patient.
- Dielectrophoresis
-
(DEP). A phenomenon in which a force is exerted on a dielectric particle when it is subjected to a non-uniform electric field. As biological cells have diverse dielectric properties, DEP can be used to manipulate, transport, separate and sort different types of particles (for example, circulating tumour cells).
- Digital PCR
-
A refinement of conventional PCR methods that can be used to directly quantify and clonally amplify nucleic acids, including DNA, cDNA or RNA that occur at very low frequencies.
- Epithelial-to-mesenchymal transition
-
(EMT). Conversion from an epithelial to a mesenchymal phenotype, which is a normal component of embryonic development. In carcinomas, this transformation results in altered cell morphology, the expression of mesenchymal proteins and increased invasiveness.
- Leukapheresis
-
A process by which a large amount of blood is withdrawn from a vein, white blood cells and circulating tumour cells are selectively removed, and the remaining blood (red blood cells in platelet- and leukocyte-poor plasma) is transfused back into the donor.
- Microfluidic devices
-
The integration of one or different laboratory functions on a single chip of only millimetres to a few square centimetres in size, in which extremely small fluid volumes (down to <picolitres) are handled.
- Parallel progression
-
Tumour cells leave the primary tumour and home to secondary sites many years before the diagnosis and surgical resection of the primary tumour. These disseminated tumour cells can develop mutations that are independent from the mutational landscape of the primary tumour.
- Photoacoustic flow cytometry
-
The irradiation of individual cells in blood and lymph flow with one or a few focused laser beams operating at different wavelengths, followed by the use of an ultrasound transducer attached to the skin to record laser-induced acoustic waves.
- Tumour, node, metastasis cancer staging
-
(TNM cancer staging). A staging system for classifying cancers that was originally developed by the American Joint Committee on Cancer (AJCC) and that grades cancer by tumour, lymph node and metastatic status.
Rights and permissions
About this article
Cite this article
Alix-Panabières, C., Pantel, K. Challenges in circulating tumour cell research. Nat Rev Cancer 14, 623–631 (2014). https://doi.org/10.1038/nrc3820
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3820