Key Points
-
Small-animal imaging in cancer research is a dynamic research field, with tremendous progress that has resulted from substantial developments in small-animal imaging techniques, specific imaging probes and advances in innovative animal tumour models.
-
Multimodal small-animal imaging techniques provide complementary and therefore more complete information (as one technique can compensate for the weaker characteristics of others).
-
Molecular imaging probes can be applied for many different purposes in cancer research, including visualization of tumour cell and extracellular matrix characteristics, diagnosis, staging, therapy selection, therapy and monitoring of therapy response.
-
Well-defined model systems and study designs are needed to bridge the gap between oncological in vitro studies and clinical application. Two stages in the translation of in vitro results can be distinguished: the first step comprises translation from the laboratory bench to animal models, the second step involves translation from animal models to the clinic. Research questions related to each stage ask for different models and matching imaging techniques to get the most reliable answers.
-
Increased knowledge of cancer type-specific genes has supported the generation of genetically engineered mouse models that capture both the cell-intrinsic and cell-extrinsic factors that drive organ-specific cancer development and the progression towards metastatic disease.
-
'Close to patient' models, such as patient-derived xenografts and reconstituted 'humanized' xenografts, as well as patient-derived ex vivo organoid three-dimensional cultures and tissue-slice systems, aim to capture the unique patient-specific tumour microenvironment and to sustain tumour heterogeneity.
-
Careful selection of the most appropriate model system and best (multimodal) imaging modalities, as well as an optimal study design, are crucial decision points that determine the translational impact of the study.
-
Close collaboration of different disciplines, specific training of preclinical and basic researchers, harmonization of protocols and stricter publication guidelines are needed and will help to further improve the translation of results from the small-animal imaging research field into the clinic.
Abstract
Recent developments and improvements of multimodal imaging methods for use in animal research have substantially strengthened the options of in vivo visualization of cancer-related processes over time. Moreover, technological developments in probe synthesis and labelling have resulted in imaging probes with the potential for basic research, as well as for translational and clinical applications. In addition, more sophisticated cancer models are available to address cancer-related research questions. This Review gives an overview of developments in these three fields, with a focus on imaging approaches in animal cancer models and how these can help the translation of new therapies into the clinic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cook, N., Jodrell, D. I. & Tuveson, D. A. Predictive in vivo animal models and translation to clinical trials. Drug Discov. Today 17, 253–260 (2012).
Heyer, J., Kwong, L. N., Lowe, S. W. & Chin, L. Non-germline genetically engineered mouse models for translational cancer research. Nature Rev. Cancer 10, 470–480 (2010).
Langdon, S. P. Animal modeling of cancer pathology and studying tumor response to therapy. Curr. Drug Targets 13, 1535–1547 (2012).
Tentler, J. J. et al. Patient-derived tumour xenografts as models for oncology drug development. Nature Rev. Clin. Oncol. 9, 338–350 (2012). This review highlights the opportunities and limitations of PDX models in cancer drug development and describes concepts regarding predictive biomarker development and future applications.
Konantz, M. et al. Zebrafish xenografts as a tool for in vivo studies on human cancer. Ann. N. Y. Acad. Sci. 1266, 124–137 (2012).
Leong, H. S., Chambers, A. F. & Lewis, J. D. Assessing cancer cell migration and metastatic growth in vivo in the chick embryo using fluorescence intravital imaging. Methods Mol. Biol. 872, 1–14 (2012).
Mimeault, M. & Batra, S. K. Emergence of zebrafish models in oncology for validating novel anticancer drug targets and nanomaterials. Drug Discov. Today 18, 128–140 (2012).
Vaira, V. et al. Preclinical model of organotypic culture for pharmacodynamic profiling of human tumors. Proc. Natl Acad. Sci. USA 107, 8352–8356 (2010).
van der Kuip, H. et al. Short term culture of breast cancer tissues to study the activity of the anticancer drug taxol in an intact tumor environment. BMC Cancer 6, 86 (2006).
Graves, E. E., Weissleder, R. & Ntziachristos, V. Fluorescence molecular imaging of small animal tumor models. Curr. Mol. Med. 4, 419–430 (2004).
Massoud, T. F. & Gambhir, S. S. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 17, 545–580 (2003).
Weissleder, R. & Mahmood, U. Molecular imaging. Radiology 219, 316–333 (2001).
Timpson, P., McGhee, E. J. & Anderson, K. I. Imaging molecular dynamics in vivo—from cell biology to animal models. J. Cell Sci. 124, 2877–2890 (2011).
Ntziachristos, V. Going deeper than microscopy: the optical imaging frontier in biology. Nature Methods 7, 603–614 (2010).
Massoud, T. F. & Gambhir, S. S. Integrating noninvasive molecular imaging into molecular medicine: an evolving paradigm. Trends Mol. Med. 13, 183–191 (2007).
Pichler, B. J., Wehrl, H. F. & Judenhofer, M. S. Latest advances in molecular imaging instrumentation. J. Nucl. Med. 49 (Suppl 2), 5S–23S (2008).
Condeelis, J. & Weissleder, R. In vivo imaging in cancer. Cold Spring Harb. Perspect. Biol. 2, a003848 (2010). This paper gives an overview of current macroscopic and microscopic imaging technologies aimed at the translation of basic molecular insight at the single cell level to clinical applications.
Cai, W. & Chen, X. Nanoplatforms for targeted molecular imaging in living subjects. Small 3, 1840–1854 (2007).
Goldenberg, D. M., Rossi, E. A., Sharkey, R. M., McBride, W. J. & Chang, C. H. Multifunctional antibodies by the Dock-and-Lock method for improved cancer imaging and therapy by pretargeting. J. Nucl. Med. 49, 158–163 (2008).
Schottelius, M. & Wester, H. J. Molecular imaging targeting peptide receptors. Methods 48, 161–177 (2009).
Zhang, Z. et al. Activatable molecular systems using homologous near-infrared fluorescent probes for monitoring enzyme activities in vitro, in cellulo, and in vivo. Mol. Pharm. 6, 416–427 (2009).
Lofblom, J. et al. Affibody molecules: engineered proteins for therapeutic, diagnostic and biotechnological applications. FEBS Lett. 584, 2670–2680 (2010).
Olafsen, T. & Wu, A. M. Antibody vectors for imaging. Semin. Nucl. Med. 40, 167–181 (2010).
Godin, B., Tasciotti, E., Liu, X., Serda, R. E. & Ferrari, M. Multistage nanovectors: from concept to novel imaging contrast agents and therapeutics. Acc. Chem. Res. 44, 979–989 (2011).
Jokerst, J. V. & Gambhir, S. S. Molecular imaging with theranostic nanoparticles. Acc. Chem. Res. 44, 1050–1060 (2011).
Devoogdt, N. et al. Molecular imaging using nanobodies: a case study. Methods Mol. Biol. 911, 559–567 (2012).
Gulyas, B. & Halldin, C. New PET radiopharmaceuticals beyond FDG for brain tumor imaging. Q. J. Nucl. Med. Mol. Imag. 56, 173–190 (2012).
Laverman, P., Sosabowski, J. K., Boerman, O. C. & Oyen, W. J. Radiolabelled peptides for oncological diagnosis. Eur. J. Nucl. Med. Mol. Imag. 39 (Suppl. 1), S78–S92 (2012).
Olafsen, T., Sirk, S. J., Olma, S., Shen, C. K. & Wu, A. M. ImmunoPET using engineered antibody fragments: fluorine-18 labeled diabodies for same-day imaging. Tumour Biol. 33, 669–677 (2012).
Tran Cao, H. S. et al. Tumor-specific fluorescence antibody imaging enables accurate staging laparoscopy in an orthotopic model of pancreatic cancer. Hepatogastroenterology 59, 1994–1999 (2012).
Weissleder, R. & Pittet, M. J. Imaging in the era of molecular oncology. Nature 452, 580–589 (2008).
Arosio, D., Casagrande, C. & Manzoni, L. Integrin-mediated drug delivery in cancer and cardiovascular diseases with peptide-functionalized nanoparticles. Curr. Med. Chem. 19, 3128–3151 (2012).
Deshpande, N., Needles, A. & Willmann, J. K. Molecular ultrasound imaging: current status and future directions. Clin. Radiol. 65, 567–581 (2010).
Lee, J. H. et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nature Med. 13, 95–99 (2007).
Kiessling, F., Fokong, S., Koczera, P., Lederle, W. & Lammers, T. Ultrasound microbubbles for molecular diagnosis, therapy, and theranostics. J. Nucl. Med. 53, 345–348 (2012).
Karmakar, A. et al. Raman spectroscopy as a detection and analysis tool for in vitro specific targeting of pancreatic cancer cells by EGF-conjugated, single-walled carbon nanotubes. J. Appl. Toxicol. 32, 365–375 (2012).
Ciarlo, M. et al. Use of the semiconductor nanotechnologies “quantum dots” for in vivo cancer imaging. Recent Pat. Anticancer Drug Discov. 4, 207–215 (2009).
Kiessling, F. Science to practice: the dawn of molecular US imaging for clinical cancer imaging. Radiology 256, 331–333 (2010).
Kluza, E. et al. Dual-targeting of αvβ3 and galectin-1 improves the specificity of paramagnetic/fluorescent liposomes to tumor endothelium in vivo. J. Control Release 158, 207–214 (2012).
Cheng, Z., Al Zaki, A., Hui, J. Z., Muzykantov, V. R. & Tsourkas, A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science 338, 903–910 (2012).
Martic-Kehl, M. I., Schibli, R. & Schubiger, P. A. Can animal data predict human outcome? Problems and pitfalls of translational animal research. Eur. J. Nucl. Med. Mol. Imaging 39, 1492–1496 (2012).
van der Worp, H. B. et al. Can animal models of disease reliably inform human studies? PLoS Med. 7, e1000245 (2010).
Begley, C. G. & Ellis, L. M. Drug development: Raise standards for preclinical cancer research. Nature 483, 531–533 (2012).
Perel, P. et al. Comparison of treatment effects between animal experiments and clinical trials: systematic review. BMJ 334, 197 (2007).
Hackam, D. G. & Redelmeier, D. A. Translation of research evidence from animals to humans. JAMA 296, 1731–1732 (2006).
Koba, W., Jelicks, L. A. & Fine, E. J. MicroPET/SPECT/CT imaging of small animal models of disease. Am. J. Pathol. 182, 319–324 (2013).
de Kemp, R. A., Epstein, F. H., Catana, C., Tsui, B. M. & Ritman, E. L. Small-animal molecular imaging methods. J. Nucl. Med. 5, (Suppl 1), 18S–32S (2010).
Shcherbakova, D. M. & Verkhusha, V. V. Near-infrared fluorescent proteins for multicolor in vivo imaging. Nature Methods 10, 751–754 (2013).
van Dam, G. M. et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nature Med. 17, 1315–1319 (2011).
Brouwer, O. R. et al. Comparing the hybrid fluorescent-radioactive tracer indocyanine green-99mTc-nanocolloid with 99mTc-nanocolloid for sentinel node identification: a validation study using lymphoscintigraphy and SPECT/CT. J. Nucl. Med. 53, 1034–1040 (2012).
Buckle, T., Brouwer, O. R., Valdes Olmos, R. A., van der Poel, H. G. & van Leeuwen, F. W. Relationship between intraprostatic tracer deposits and sentinel lymph node mapping in prostate cancer patients. J. Nucl. Med. 53, 1026–1033 (2012).
Buckle, T., Chin, P. T. & van Leeuwen, F. W. (Non-targeted) radioactive/fluorescent nanoparticles and their potential in combined pre- and intraoperative imaging during sentinel lymph node resection. Nanotechnology 21, 482001 (2010).
Filonov, G. S. et al. Bright and stable near-infrared fluorescent protein for in vivo imaging. Nature Biotech. 29, 757–761 (2011).
Hoffman, R. M. Cellular and subcellular imaging in live mice using fluorescent proteins. Curr. Pharm. Biotechnol. 13, 537–544 (2012).
Kelkar, M. & De, A. Bioluminescence based in vivo screening technologies. Curr. Opin. Pharmacol. 12, 592–600 (2012).
O'Neill, K., Lyons, S. K., Gallagher, W. M., Curran, K. M. & Byrne, A. T. Bioluminescent imaging: a critical tool in pre-clinical oncology research. J. Pathol. 220, 317–327 (2010).
Kim, J. B. et al. Non-invasive detection of a small number of bioluminescent cancer cells in vivo. PLoS ONE 5, e9364 (2010).
Hoffman, R. M. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nature Rev. Cancer 5, 796–806 (2005).
Timpson, P. et al. Spatial regulation of RhoA activity during pancreatic cancer cell invasion driven by mutant p53. Cancer Res. 71, 747–757 (2011).
Kiessling, F. & Pichler, B. Small animal imaging. Basics and practical guide., (Springer, 2012).
Rossin, R. et al. In vivo chemistry for pretargeted tumor imaging in live mice. Angew. Chem. Int. Ed. Engl. 49, 3375–3378 (2010).
Elsabahy, M. & Wooley, K. L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 41, 2545–2561 (2012).
Petros, R. A. & DeSimone, J. M. Strategies in the design of nanoparticles for therapeutic applications. Nature Rev. Drug Discov. 9, 615–627 (2010).
Brader, P., Serganova, I. & Blasberg, R. G. Noninvasive molecular imaging using reporter genes. J. Nucl. Med. 54, 167–172 (2013).
Ray, P. & De, A. Reporter gene imaging in therapy and diagnosis. Theranostics 2, 333–334 (2012).
Pool, S. E., ten Hagen, T. L., Koelewijn, S., de Jong, M. & Koning, G. A. Multimodality imaging of somatostatin receptor-positive tumors with nuclear and bioluminescence imaging. Mol. Imag. 11, 27–32 (2012).
Schroeder, R. P., van Weerden, W. M., Bangma, C., Krenning, E. P. & de Jong, M. Peptide receptor imaging of prostate cancer with radiolabelled bombesin analogues. Methods 48, 200–204 (2009).
Stelter, L. et al. An orthotopic model of pancreatic somatostatin receptor (SSTR)-positive tumors allows bimodal imaging studies using 3T MRI and animal PET-based molecular imaging of SSTR expression. Neuroendocrinology 87, 233–242 (2008).
Kang, J. H. & Chung, J. K. Molecular-genetic imaging based on reporter gene expression. J. Nucl. Med. 49, (Suppl 2) 164S–179S (2008).
Chua, S., Gnanasegaran, G. & Cook, G. J. Miscellaneous cancers (lung, thyroid, renal cancer, myeloma, and neuroendocrine tumors): role of SPECT and PET in imaging bone metastases. Semin. Nucl. Med. 39, 416–430 (2009).
Vandsburger, M. H., Radoul, M., Cohen, B. & Neeman, M. MRI reporter genes: applications for imaging of cell survival, proliferation, migration and differentiation. NMR Biomed. 24, 872–884 (2012).
Cohen, B. et al. MRI detection of transcriptional regulation of gene expression in transgenic mice. Nature Med. 13, 498–503 (2007).
Gilad, A. A. et al. MRI reporter genes. J. Nucl. Med. 49, 1905–1908 (2008).
Avni, R., Cohen, B. & Neeman, M. Hypoxic stress and cancer: imaging the axis of evil in tumor metastasis. NMR Biomed. 24, 569–581 (2011).
Cai, W., Niu, G. & Chen, X. Imaging of integrins as biomarkers for tumor angiogenesis. Curr. Pharm. Des. 14, 2943–2973 (2008).
Roivainen, A., Jalkanen, S. & Nanni, C. Gallium-labelled peptides for imaging of inflammation. Eur. J. Nucl. Med. Mol. Imag. 39 (Suppl. 1), S68–S77 (2012).
de Jong, M., Breeman, W. A., Kwekkeboom, D. J., Valkema, R. & Krenning, E. P. Tumor imaging and therapy using radiolabeled somatostatin analogues. Acc. Chem. Res. 42, 873–880 (2009). This study is an example of the theranostic concept using radiopeptides.
Ambrosini, V., Fani, M., Fanti, S., Forrer, F. & Maecke, H. R. Radiopeptide imaging and therapy in Europe. J. Nucl. Med. 52, (Suppl 2), 42S–55S (2011).
Kwekkeboom, D. J. et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J. Clin. Oncol. 26, 2124–2130 (2008).
Louie, A. Multimodality imaging probes: design and challenges. Chem. Rev. 110, 3146–3195 (2010).
Zhang, L. et al. Nanoparticles in medicine: therapeutic applications and developments. Clin. Pharmacol. Ther. 83, 761–769 (2008).
Bednar, B. & Ntziachristos, V. Opto-acoustic imaging of drug discovery biomarkers. Curr. Pharm. Biotechnol. 13, 2117–2127 (2012).
Kenny, G. D. et al. Multifunctional receptor-targeted nanocomplexes for magnetic resonance imaging and transfection of tumours. Biomaterials 33, 7241–7250 (2012).
Xu, C. & Zhao, W. Nanoparticle-based monitoring of stem cell therapy. Theranostics 3, 616–617 (2013).
Agasti, S. S. et al. Dual imaging and photoactivated nanoprobe for controlled cell tracking. Small 9, 222–227 (2013).
Kircher, M. F., Gambhir, S. S. & Grimm, J. Noninvasive cell-tracking methods. Nature Rev. Clin. Oncol. 8, 677–688 (2011). This paper provides an overview of the basic principles of cell-tracking methods.
Srinivas, M. et al. Imaging of cellular therapies. Adv. Drug Deliv. Rev. 62, 1080–1093 (2010).
Rygaard, J. & Povlsen, C. O. Heterotransplantation of a human malignant tumour to “Nude” mice. Acta Pathol. Microbiol. Scand. 77, 758–760 (1969).
Sausville, E. A. & Burger, A. M. Contributions of human tumor xenografts to anticancer drug development. Cancer Res. 66, 3351–3354, (discussion 3354) (2006).
Moro, M. et al. Patient-derived xenografts of non small cell lung cancer: resurgence of an old model for investigation of modern concepts of tailored therapy and cancer stem cells. J. Biomed. Biotechnol. http://dx.doi.org/10.1155/2012/568567 (2012).
Ito, R., Takahashi, T., Katano, I. & Ito, M. Current advances in humanized mouse models. Cell. Mol. Immunol. 9, 208–214 (2012).
Zhao, H., Nolley, R., Chen, Z. & Peehl, D. M. Tissue slice grafts: an in vivo model of human prostate androgen signaling. Am. J. Pathol. 177, 229–239 (2010).
Fidler, I. J. Host and tumour factors in cancer metastasis. Eur. J. Clin. Invest. 20, 481–486 (1990).
Pettaway, C. A. et al. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin. Cancer Res. 2, 1627–1636 (1996).
Hoffman, R. M. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest. New Drugs 17, 343–359 (1999).
Fidler, I. J. Biological heterogeneity of cancer: implication to therapy. Hum. Vaccin Immunother. 8, 1141–1142 (2012).
Langley, R. R. & Fidler, I. J. The seed and soil hypothesis revisited—the role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 128, 2527–2535 (2011). This review describes the favourable tumour–stroma interactions that underlie the organ-preference pattern of metastasis that is observed in cancer.
Scheer, N. & Roland Wolf, C. Xenobiotic receptor humanized mice and their utility. Drug Metab. Rev. 45, 110–121 (2012).
Friedl, P. & Alexander, S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147, 992–1009 (2011).
Friedl, P., Locker, J., Sahai, E. & Segall, J. E. Classifying collective cancer cell invasion. Nature Cell Biol. 14, 777–783 (2012). This is a proposed framework for studying collective invasion.
Neeman, M., Gilad, A. A., Dafni, H. & Cohen, B. Molecular imaging of angiogenesis. J. Magn. Reson. Imag. 25, 1–12 (2007).
Vandoorne, K., Addadi, Y. & Neeman, M. Visualizing vascular permeability and lymphatic drainage using labeled serum albumin. Angiogenesis 13, 75–85 (2010).
Nemati, F. et al. Establishment and characterization of a panel of human uveal melanoma xenografts derived from primary and/or metastatic tumors. Clin. Cancer Res. 16, 2352–2362 (2010).
Press, J. Z. et al. Xenografts of primary human gynecological tumors grown under the renal capsule of NOD/SCID mice show genetic stability during serial transplantation and respond to cytotoxic chemotherapy. Gynecol. Oncol. 110, 256–264 (2008).
van Weerden, W. M. & van Steenbrugge, G. J. Human prostate tumor xenografts as representative models for clinical prostate cancer. Urol. Oncol. 2, 122–125 (1996).
Jin, K. et al. Patient-derived human tumour tissue xenografts in immunodeficient mice: a systematic review. Clin. Transl. Oncol. 12, 473–480 (2010).
Fu, X., Guadagni, F. & Hoffman, R. M. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc. Natl Acad. Sci. USA 89, 5645–5649 (1992).
Fu, X. Y., Besterman, J. M., Monosov, A. & Hoffman, R. M. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc. Natl Acad. Sci. USA 88, 9345–9349 (1991).
Metildi, C. A. et al. Fluorescently labeled chimeric anti-CEA antibody improves detection and resection of human colon cancer in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J. Surg. Oncol. 109, 451–458 (2014).
Berger, D. P., Winterhalter, B. R. & Fiebig, H. H. in Immunodeficient mice in oncology, Vol. 42 (eds Fiebig, H. H. & Berger, D. P.) 23–46 (Karger, 1992).
Vlietstra, R. J., van Alewijk, D. C., Hermans, K. G., van Steenbrugge, G. J. & Trapman, J. Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res. 58, 2720–2723 (1998).
Kerbel, R. S. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived-but they can be improved. Cancer Biol. Ther. 2, S134–S139 (2003).
Fichtner, I. et al. Establishment of patient-derived non-small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin. Cancer Res. 14, 6456–6468 (2008).
Hermans, K. G. et al. TMPRSS2:ERG fusion by translocation or interstitial deletion is highly relevant in androgen-dependent prostate cancer, but is bypassed in late-stage androgen receptor-negative prostate cancer. Cancer Res. 66, 10658–10663 (2006).
DeRose, Y. S. et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nature Med. 17, 1514–1520 (2011).
Bankert, R. B. et al. Humanized mouse model of ovarian cancer recapitulates patient solid tumor progression, ascites formation, and metastasis. PLoS ONE 6, e24420 (2011).
Garcia, S. & Freitas, A. A. Humanized mice: current states and perspectives. Immunol. Lett. 146, 1–7 (2012). In this review, an update is provided on the newest versions of humanized mice bearing a human immune system. It describes the limitations of these mice and the current approaches to overcome these limitations.
Shultz, L. D., Brehm, M. A., Bavari, S. & Greiner, D. L. Humanized mice as a preclinical tool for infectious disease and biomedical research. Ann. NY Acad. Sci. 1245, 50–54 (2011).
Shultz, L. D., Ishikawa, F. & Greiner, D. L. Humanized mice in translational biomedical research. Nature Rev. Immunol. 7, 118–130 (2007).
Montecinos, V. P., Godoy, A., Hinklin, J., Vethanayagam, R. R. & Smith, G. J. Primary xenografts of human prostate tissue as a model to study angiogenesis induced by reactive stroma. PLoS ONE 7, e29623 (2012).
Wang, Y. et al. Development and characterization of efficient xenograft models for benign and malignant human prostate tissue. Prostate 64, 149–159 (2005).
Simpson-Abelson, M. R. et al. Long-term engraftment and expansion of tumor-derived memory T cells following the implantation of non-disrupted pieces of human lung tumor into NOD-scid IL2Rγ(null) mice. J. Immunol. 180, 7009–7018 (2008).
Ito, R. et al. Antigen-specific antibody production of human B cells in NOG mice reconstituted with the human immune system. Curr. Top. Microbiol. Immunol. 324, 95–107 (2008).
Ji, M., Jin, X., Phillips, P. & Yi, S. A humanized mouse model to study human immune response in xenotransplantation. Hepatobiliary Pancreat. Dis. Int. 11, 494–498 (2012).
van Miltenburg, M. H. & Jonkers, J. Using genetically engineered mouse models to validate candidate cancer genes and test new therapeutic approaches. Curr. Opin. Genet. Dev. 22, 21–27 (2012). This review highlights the recent technological advances in modelling cancer in GEMMs, their usefulness and the challenges encountered.
Walrath, J. C., Hawes, J. J., Van Dyke, T. & Reilly, K. M. Genetically engineered mouse models in cancer research. Adv. Cancer Res. 106, 113–164 (2010).
Eklund, L., Bry, M. & Alitalo, K. Mouse models for studying angiogenesis and lymphangiogenesis in cancer. Mol. Oncol. 7, 259–282 (2013).
Firestone, B. The challenge of selecting the 'right' in vivo oncology pharmacology model. Curr. Opin. Pharmacol. 10, 391–396 (2010).
Schaffer, B. S. et al. Immune competency of a hairless mouse strain for improved preclinical studies in genetically engineered mice. Mol. Cancer Ther. 9, 2354–2364 (2010).
Naumov, G. N. et al. Cellular expression of green fluorescent protein, coupled with high-resolution in vivo videomicroscopy, to monitor steps in tumor metastasis. J. Cell Sci. 112, 1835–1842 (1999).
Yang, M. et al. Direct external imaging of nascent cancer, tumor progression, angiogenesis, and metastasis on internal organs in the fluorescent orthotopic model. Proc. Natl Acad. Sci. USA 99, 3824–3829 (2002).
Yang, M., Jiang, P. & Hoffman, R. M. Whole-body subcellular multicolor imaging of tumor-host interaction and drug response in real time. Cancer Res. 67, 5195–5200 (2007).
Hoffman, R. M. Imaging cancer dynamics in vivo at the tumor and cellular level with fluorescent proteins. Clin. Exp. Metastasis 26, 345–355 (2009).
Yamamoto, N., Tsuchiya, H. & Hoffman, R. M. Tumor imaging with multicolor fluorescent protein expression. Int. J. Clin. Oncol. 16, 84–91 (2011).
Nothdurft, R., Sarder, P., Bloch, S., Culver, J. & Achilefu, S. Fluorescence lifetime imaging microscopy using near-infrared contrast agents. J. Microsc. 247, 202–207 (2012).
Lin, M. Z. et al. Autofluorescent proteins with excitation in the optical window for intravital imaging in mammals. Chem. Biol. 16, 1169–1179 (2009).
Morozova, K. S. et al. Far-red fluorescent protein excitable with red lasers for flow cytometry and superresolution STED nanoscopy. Biophys. J. 99, L13–15 (2010).
Shcherbo, D. et al. Far-red fluorescent tags for protein imaging in living tissues. Biochem. J. 418, 567–574 (2009).
Shcherbo, D. et al. Near-infrared fluorescent proteins. Nature Methods 7, 827–829 (2010). This paper describes the development of NIRF proteins with red-shifted emission maxima and high photostability for in vivo imaging.
Strack, R. L. et al. A rapidly maturing far-red derivative of DsRed-Express2 for whole-cell labeling. Biochemistry 48, 8279–8281 (2009).
Beerling, E., Ritsma, L., Vrisekoop, N., Derksen, P. W. & van Rheenen, J. Intravital microscopy: new insights into metastasis of tumors. J. Cell Sci. 124, 299–310 (2011).
Ritsma, L. et al. Intravital microscopy through an abdominal imaging window reveals a pre-micrometastasis stage during liver metastasis. Sci. Transl. Med. 4, 158ra145 (2012).
Souris, J. S., Hickson, J. A., Msezane, L., Rinker-Schaeffer, C. W. & Chen, C. T. Flexible peritoneal windows for quantitative fluorescence and bioluminescence preclinical imaging. Mol. Imag. 12, 28–38 (2013).
Tanaka, K. et al. Intravital dual-colored visualization of colorectal liver metastasis in living mice using two photon laser scanning microscopy. Microsc. Res. Tech. 75, 307–315 (2012).
Amoh, Y., Katsuoka, K. & Hoffman, R. M. Color-coded fluorescent protein imaging of angiogenesis: the AngioMouse models. Curr. Pharm. Des. 14, 3810–3819 (2008).
van der Horst, G. et al. Real-time cancer cell tracking by bioluminescence in a preclinical model of human bladder cancer growth and metastasis. Eur. Urol. 60, 337–343 (2011).
Durupt, F. et al. The chicken chorioallantoic membrane tumor assay as model for qualitative testing of oncolytic adenoviruses. Cancer Gene Ther. 19, 58–68 (2012).
Harma, V. et al. A comprehensive panel of three-dimensional models for studies of prostate cancer growth, invasion and drug responses. PLoS ONE 5, e10431 (2010).
Sato, T. & Clevers, H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340, 1190–1194 (2013).
Sachs, N. & Clevers, H. Organoid cultures for the analysis of cancer phenotypes. Curr. Opin. Genet. Dev. 24C, 68–73 (2014).
Fiebig, H. H. et al. Gene signatures developed from patient tumor explants grown in nude mice to predict tumor response to 11 cytotoxic drugs. Cancer Genomics Proteomics 4, 197–209 (2007).
Parrish, A. R., Gandolfi, A. J. & Brendel, K. Precision-cut tissue slices: applications in pharmacology and toxicology. Life Sci. 57, 1887–1901 (1995).
Pickl, M. & Ries, C. H. Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene 28, 461–468 (2009).
Willoughby, H. W., Maughan, G. B., Tremblay, P. C. & Wood, N. Determination of individual human tumour sensitivity to antitumour agents by tissue-slice incubation. Can. J. Surg. 14, 406–409 (1971).
Zschenker, O., Streichert, T., Hehlgans, S. & Cordes, N. Genome-wide gene expression analysis in cancer cells reveals 3D growth to affect ECM and processes associated with cell adhesion but not DNA repair. PLoS ONE 7, e34279 (2012).
Keyaerts, M. et al. Inhibition of firefly luciferase by general anesthetics: effect on in vitro and in vivo bioluminescence imaging. PLoS ONE 7, e30061 (2012).
Fueger, B. J. et al. Impact of animal handling on the results of 18F-FDG PET studies in mice. J. Nucl. Med. 47, 999–1006 (2006).
Emonds, K. M. et al. Do androgens control the uptake of 18F-FDG, 11C-choline and 11C-acetate in human prostate cancer cell lines? Eur. J. Nucl. Med. Mol. Imaging 38, 1842–1853 (2011).
Gros, S. J. et al. Complementary use of fluorescence and magnetic resonance imaging of metastatic esophageal cancer in a novel orthotopic mouse model. Int. J. Cancer 126, 2671–2681 (2010).
Kosaka, N., Bernardo, M., Mitsunaga, M., Choyke, P. L. & Kobayashi, H. MR and optical imaging of early micrometastases in lymph nodes: triple labeling with nano-sized agents yielding distinct signals. Contrast Media Mol. Imag. 7, 247–253 (2012).
Krupnick, A. S. et al. Quantitative monitoring of mouse lung tumors by magnetic resonance imaging. Nature Protoc. 7, 128–142 (2012).
Wolf, G. & Abolmaali, N. Preclinical molecular imaging using PET and MRI. Recent Results Cancer Res. 187, 257–310 (2013).
Pittet, M. J. & Weissleder, R. Intravital imaging. Cell 147, 983–991 (2011). This is an overview of state-of-the-art intravital imaging techniques and emerging technologies in this field.
Workman, P. et al. Guidelines for the welfare and use of animals in cancer research. Br. J. Cancer 102, 1555–1577 (2010).
Festing, M. F. Improving the design and analysis of animal experiments: a personal odyssey. Altern. Lab. Anim. 37 (Suppl. 2), 75–81 (2009).
Festing, M. F. Inbred strains should replace outbred stocks in toxicology, safety testing, and drug development. Toxicol. Pathol. 38, 681–690 (2010).
Klaunberg, B. A. & Davis, J. A. Considerations for laboratory animal imaging center design and setup. ILAR J. 49, 4–16 (2008).
Maina, T. et al. Species differences of bombesin analog interactions with GRP-R define the choice of animal models in the development of GRP-R-targeting drugs. J. Nucl. Med. 46, 823–830 (2005).
Wagner, P. D. & Srivastava, S. New paradigms in translational science research in cancer biomarkers. Transl. Res. 159, 343–353 (2012). This is a perspective on cancer biomarker translation.
Roberts, S. F., Fischhoff, M. A., Sakowski, S. A. & Feldman, E. L. Perspective: Transforming science into medicine: how clinician-scientists can build bridges across research's valley of death. Acad. Med. 87, 266–270 (2012).
Caponigro, G. & Sellers, W. R. Advances in the preclinical testing of cancer therapeutic hypotheses. Nature Rev. Drug Discov. 10, 179–187 (2011).
Feitsma, H. & Cuppen, E. Zebrafish as a cancer model. Mol. Cancer Res. 6, 685–694 (2008).
Ale, A. et al. FMT-XCT: in vivo animal studies with hybrid fluorescence molecular tomography-X-ray computed tomography. Nature Methods 9, 615–620 (2012).
Afaq, A. & Akin, O. Imaging assessment of tumor response: past, present and future. Future Oncol. 7, 669–677 (2011).
Acknowledgements
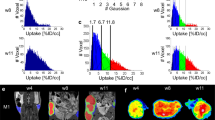
The authors thank the members of their laboratories for their critical comments and helpful discussions. The authors thank the biotechnicians in the groups for many years of dedicated work 'behind the scenes' to propagate precious PDX models valuable to many research projects, as well as for the many imaging studies performed; the authors thank the SPECTRIM group at Erasmus MC, The Netherlands, for providing Figures 2a and 2b. The authors apologize to those whose work is not cited owing to space limitations. Part of the authors' research is funded by Erasmus MC grants, grants from the SUWO (Stichting Urologisch Wetenschappelijk Onderzoek), from the Innovative Medicines Initiative (IMI) Joint Undertaking ('PREDECT', grant agreement number 115188) by EU FP7 and EFPIA companies, a grant from the 'Lijf en Leven' foundation ('DIVERS'), equipment grants (91111012 and 91105015) from the Dutch Organization of Scientific Research (NWO), a grant from the Dutch Cancer Society (KWF; EMCR 2008-4037) and a grant from EU FP7 ITN PITN-GA-2012-317019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
de Jong, M., Essers, J. & van Weerden, W. Imaging preclinical tumour models: improving translational power. Nat Rev Cancer 14, 481–493 (2014). https://doi.org/10.1038/nrc3751
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3751