Key Points
-
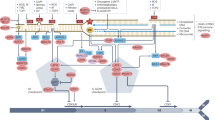
DNA repair defects are targets for chemotherapy drugs.
-
DNA damage response (DDR) genes are also targets for resistance mechanisms that are acquired during chemotherapy treatment.
-
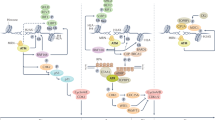
To enhance chemotherapy response, the DDR may be targeted by reactivation of p53, by inhibition of cell cycle checkpoints or by inhibition of DNA repair processes.
-
Therapy resistance of homologous recombination (HR)-deficient tumours may be caused by genetic reversion of the HR defect, by residual HR activity, by rewiring of DNA repair pathways or by tumour heterogeneity.
-
Robust biomarkers are required to maximize the effectiveness of therapy targeting HR deficiency.
-
The best possible treatments might involve combinations of chemotherapy drugs and/or targeted therapeutics to eradicate tumours before resistant tumour cell clones arise.
Abstract
Tumours with specific DNA repair defects can be completely dependent on back-up DNA repair pathways for their survival. This dependence can be exploited therapeutically to induce synthetic lethality in tumour cells. For instance, homologous recombination (HR)-deficient tumours can be effectively targeted by DNA double-strand break-inducing agents. However, not all HR-defective tumours respond equally well to this type of therapy. Tumour cells may acquire resistance by invoking biochemical mechanisms that reduce drug action or by acquiring additional alterations in DNA damage response pathways. A thorough understanding of these processes is important for predicting treatment response and for the development of novel treatment strategies that prevent the emergence of therapy-resistant tumours.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
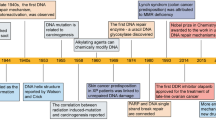
Ciccia, A. & Elledge, S. J. The DNA damage response: making it safe to play with knives. Mol. Cell 40, 179–204 (2010). An extensive review of factors and pathways involved in the DDR.
Bergink, S. & Jentsch, S. Principles of ubiquitin and SUMO modifications in DNA repair. Nature 458, 461–467 (2009).
Moynahan, M. E., Chiu, J. W., Koller, B. H. & Jasin, M. Brca1 controls homology-directed DNA repair. Mol. Cell 4, 511–518 (1999).
Moynahan, M. E., Pierce, A. J. & Jasin, M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol. Cell 7, 263–272 (2001).
Miki, Y. et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266, 66–71 (1994).
Wooster, R. et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 378, 789–792 (1995).
Fishel, R. et al. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 75, 1027–1038 (1993).
Leach, F. S. et al. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell 75, 1215–1225 (1993).
Papadopoulos, N. et al. Mutation of a mutL homolog in hereditary colon cancer. Science 263, 1625–1629 (1994).
Lynch, H. T. et al. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin. Genet. 76, 1–18 (2009).
Martin, L. P., Hamilton, T. C. & Schilder, R. J. Platinum resistance: the role of DNA repair pathways. Clin. Cancer Res. 14, 1291–1295 (2008).
Neijenhuis, S., Verwijs-Janssen, M., van den Broek, L. J., Begg, A. C. & Vens, C. Targeted radiosensitization of cells expressing truncated DNA polymerase β. Cancer Res. 70, 8706–8714 (2010).
Audeh, M. W. et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376, 245–251 (2010).
Bryant, H. E. et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434, 913–917 (2005).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005).
Fong, P. C. et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 361, 123–134 (2009).
Tutt, A. et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 376, 235–244 (2010).
Ashworth, A., Lord, C. J. & Reis-Filho, J. S. Genetic interactions in cancer progression and treatment. Cell 145, 30–38 (2011).
Cotta-Ramusino, C. et al. A DNA damage response screen identifies RHINO, a 9-1-1 and TopBP1 interacting protein required for ATR signaling. Science 332, 1313–1317 (2011).
Menzel, T. et al. A genetic screen identifies BRCA2 and PALB2 as key regulators of G2 checkpoint maintenance. EMBO Rep. 12, 705–712 (2011).
Jirawatnotai, S. et al. A function for cyclin D1 in DNA repair uncovered by protein interactome analyses in human cancers. Nature 474, 230–234 (2011).
Martins, C. P., Brown-Swigart, L. & Evan, G. I. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell 127, 1323–1334 (2006).
Ventura, A. et al. Restoration of p53 function leads to tumour regression in vivo. Nature 445, 661–665 (2007).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007).
Feldser, D. M. et al. Stage-specific sensitivity to p53 restoration during lung cancer progression. Nature 468, 572–575 (2010).
Junttila, M. R. et al. Selective activation of p53-mediated tumour suppression in high-grade tumours. Nature 468, 567–571 (2010). References 22–26 explore the effect of p53 restoration in mouse tumour models. Activation of p53 in established tumours leads to regression (references 22–24), but it does not affect early lesions with low expression of p19ARF (references 25 and 26).
Christophorou, M. A., Ringshausen, I., Finch, A. J., Swigart, L. B. & Evan, G. I. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature 443, 214–217 (2006).
Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004).
Issaeva, N. et al. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nature Med. 10, 1321–1328 (2004).
Bykov, V. J. et al. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nature Med. 8, 282–288 (2002). References 28–30 were the first studies to describe small molecule activators of the p53 tumour suppressor.
Baylin, S. B. & Jones, P. A. A decade of exploring the cancer epigenome — biological and translational implications. Nature Rev. Cancer 11, 726–734 (2011).
Cheok, C. F., Verma, C. S., Baselga, J. & Lane, D. P. Translating p53 into the clinic. Nature Rev. Clin. Oncol. 8, 25–37 (2011).
Reinhardt, H. C., Aslanian, A. S., Lees, J. A. & Yaffe, M. B. p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell 11, 175–189 (2007). This article shows that p53-deficient tumour cells depend on the p38MAPK–MK2 pathway to prevent DNA damage-induced mitotic catastrophe.
Reinhardt, H. C. et al. DNA damage activates a spatially distinct late cytoplasmic cell-cycle checkpoint network controlled by MK2-mediated RNA stabilization. Mol. Cell 40, 34–49 (2010).
Wang, Q. et al. UCN-01: a potent abrogator of G2 checkpoint function in cancer cells with disrupted p53. J. Natl Cancer Inst. 88, 956–965 (1996).
Bartek, J. & Lukas, J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3, 421–429 (2003).
Dai, Y. & Grant, S. New insights into checkpoint kinase 1 in the DNA damage response signaling network. Clin. Cancer Res. 16, 376–383 (2010).
Liu, Q. et al. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 14, 1448–1459 (2000).
Takai, H. et al. Aberrant cell cycle checkpoint function and early embryonic death in Chk1−/− mice. Genes Dev. 14, 1439–1447 (2000).
Hirao, A. et al. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 287, 1824–1827 (2000).
Takai, H. et al. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J. 21, 5195–5205 (2002).
Bao, S. et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760 (2006).
Squatrito, M. et al. Loss of ATM/Chk2/p53 pathway components accelerates tumor development and contributes to radiation resistance in gliomas. Cancer Cell 18, 619–629 (2010).
Niida, H. et al. Cooperative functions of Chk1 and Chk2 reduce tumour susceptibility in vivo. EMBO J. 29, 3558–3570 (2010).
Jiang, H. et al. The combined status of ATM and p53 link tumor development with therapeutic response. Genes Dev. 23, 1895–1909 (2009). This article shows that mechanisms commonly used by tumours to bypass early neoplastic checkpoints — such as inactivation of TP53 or ATM — determine chemotherapeutic response.
Mir, S. E. et al. In silico analysis of kinase expression identifies WEE1 as a gatekeeper against mitotic catastrophe in glioblastoma. Cancer Cell 18, 244–257 (2010). This article shows that inhibition of WEE1 in a preclinical mouse model sensitizes glioblastomas to DNA damaging therapy without causing significant side effects.
Calabrese, C. R. et al. Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. J. Natl Cancer Inst. 96, 56–67 (2004).
Donawho, C. K. et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin. Cancer Res. 13, 2728–2737 (2007).
Evers, B. et al. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin. Cancer Res. 14, 3916–3925 (2008).
Rottenberg, S. et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl Acad. Sci. USA 105, 17079–17084 (2008).
Adhikari, S. et al. Targeting base excision repair for chemosensitization. Anticancer Agents Med. Chem. 8, 351–357 (2008).
Doles, J. et al. Suppression of Rev3, the catalytic subunit of Polζ, sensitizes drug-resistant lung tumors to chemotherapy. Proc. Natl Acad. Sci. USA 107, 20786–20791 (2010).
Xie, K., Doles, J., Hemann, M. T. & Walker, G. C. Error-prone translesion synthesis mediates acquired chemoresistance. Proc. Natl Acad. Sci. USA 107, 20792–20797 (2010). References 52 and 53 demonstrate the use of preclinical mouse models to investigate mechanisms of chemotherapy resistance, and show that suppression of TLS not only sensitizes tumours to DNA damaging therapy but also limits acquired drug resistance.
Bhaskara, S. et al. Deletion of histone deacetylase 3 reveals critical roles in S phase progression and DNA damage control. Mol. Cell 30, 61–72 (2008).
Bhaskara, S. et al. Hdac3 is essential for the maintenance of chromatin structure and genome stability. Cancer Cell 18, 436–447 (2010).
Kao, G. D. et al. Histone deacetylase 4 interacts with 53BP1 to mediate the DNA damage response. J. Cell Biol. 160, 1017–1027 (2003).
Miller, K. M. et al. Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nature Struct. Mol. Biol. 17, 1144–1151 (2010).
Fan, W. & Luo, J. SIRT1 regulates UV-induced DNA repair through deacetylating XPA. Mol. Cell 39, 247–258 (2010).
Ming, M. et al. Regulation of global genome nucleotide excision repair by SIRT1 through xeroderma pigmentosum C. Proc. Natl Acad. Sci. USA 107, 22623–22628 (2010).
Wang, R. H. et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell 14, 312–323 (2008).
Yamamori, T. et al. SIRT1 deacetylates APE1 and regulates cellular base excision repair. Nucleic Acids Res. 38, 832–845 (2010).
Yuan, Z., Zhang, X., Sengupta, N., Lane, W. S. & Seto, E. SIRT1 regulates the function of the Nijmegen breakage syndrome protein. Mol. Cell 27, 149–162 (2007).
Kaidi, A., Weinert, B. T., Choudhary, C. & Jackson, S. P. Human SIRT6 promotes DNA end resection through CtIP deacetylation. Science 329, 1348–1353 (2010).
Mostoslavsky, R. et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124, 315–329 (2006).
Wang, R. H. et al. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol. Cell 32, 11–20 (2008).
Johnson, N. et al. Compromised CDK1 activity sensitizes BRCA-proficient cancers to PARP inhibition. Nature Med. 17, 875–882 (2011).
Johnson, N. et al. Cdk1 participates in BRCA1-dependent S phase checkpoint control in response to DNA damage. Mol. Cell 35, 327–339 (2009).
Krawczyk, P. M. et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc. Natl Acad. Sci. USA 108, 9851–9856 (2011).
Gottesman, M. M., Fojo, T. & Bates, S. E. Multidrug resistance in cancer: role of ATP-dependent transporters. Nature Rev. Cancer 2, 48–58 (2002).
Olive, K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Ishida, S., McCormick, F., Smith-McCune, K. & Hanahan, D. Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell 17, 574–583 (2010).
Edwards, S. L. et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature 451, 1111–1115 (2008).
Sakai, W. et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature 451, 1116–1120 (2008). References 72 and 73 were the first studies to show that genetic reversion of BRCA mutations can cause resistance to therapy targeting HR deficiency.
Norquist, B. et al. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J. Clin. Oncol. 29, 3008–3015 (2011).
Swisher, E. M. et al. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 68, 2581–2586 (2008).
Liu, X. et al. Somatic loss of BRCA1 and p53 in mice induces mammary tumors with features of human BRCA1-mutated basal-like breast cancer. Proc. Natl Acad. Sci. USA 104, 12111–12116 (2007).
Rottenberg, S. et al. Selective induction of chemotherapy resistance of mammary tumors in a conditional mouse model for hereditary breast cancer. Proc. Natl Acad. Sci. USA 104, 12117–12122 (2007).
Ikeda, H. et al. Genetic reversion in an acute myelogenous leukemia cell line from a Fanconi anemia patient with biallelic mutations in BRCA2. Cancer Res. 63, 2688–2694 (2003).
Litman, R. et al. BACH1 is critical for homologous recombination and appears to be the Fanconi anemia gene product FANCJ. Cancer Cell 8, 255–265 (2005).
Sy, S. M., Huen, M. S. & Chen, J. PALB2 is an integral component of the BRCA complex required for homologous recombination repair. Proc. Natl Acad. Sci. USA 106, 7155–7160 (2009).
Zhang, F., Fan, Q., Ren, K. & Andreassen, P. R. PALB2 functionally connects the breast cancer susceptibility proteins BRCA1 and BRCA2. Mol. Cancer Res. 7, 1110–1118 (2009).
Zhang, F. et al. PALB2 links BRCA1 and BRCA2 in the DNA-damage response. Curr. Biol. 19, 524–529 (2009).
Xia, B. et al. Fanconi anemia is associated with a defect in the BRCA2 partner PALB2. Nature Genet. 39, 159–161 (2007).
Lo, T. F. Jr et al. Somatic mosaicism in Fanconi anemia: molecular basis and clinical significance. Eur. J. Hum. Genet. 5, 137–148 (1997).
Waisfisz, Q. et al. Spontaneous functional correction of homozygous fanconi anaemia alleles reveals novel mechanistic basis for reverse mosaicism. Nature Genet. 22, 379–383 (1999).
Levine, A. J. & Oren, M. The first 30 years of p53: growing ever more complex. Nature Rev. Cancer 9, 749–758 (2009).
Lang, G. A. et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 119, 861–872 (2004).
Olive, K. P. et al. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 119, 847–860 (2004).
Bergamaschi, D. et al. p53 polymorphism influences response in cancer chemotherapy via modulation of p73-dependent apoptosis. Cancer Cell 3, 387–402 (2003).
Irwin, M. S. et al. Chemosensitivity linked to p73 function. Cancer Cell 3, 403–410 (2003).
Song, H., Hollstein, M. & Xu, Y. p53 gain-of-function cancer mutants induce genetic instability by inactivating ATM. Nature Cell Biol. 9, 573–580 (2007).
Wang, H. et al. Allele-specific tumor spectrum in Pten knockin mice. Proc. Natl Acad. Sci. USA 107, 5142–5147 (2010).
Mendes-Pereira, A. M. et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol. Med. 1, 315–322 (2009).
Saal, L. H. et al. Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nature Genet. 40, 102–107 (2008).
Shakya, R. et al. BRCA1 tumor suppression depends on BRCT phosphoprotein binding, but not its E3 ligase activity. Science 334, 525–528 (2011).
Drost, R. et al. BRCA1 RING function is essential for tumor suppression but dispensable for therapy resistance. Cancer Cell 20, 797–809 (2011). This article shows that the BRCA1-C61G mutation in the N-terminal RING domain predisposes to HR-deficient mammary tumours in mice, but also allows rapid resistance to therapy targeting HR deficiency without evidence for genetic reversion.
Shafee, N. et al. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res. 68, 3243–3250 (2008).
ElShamy, W. M. & Livingston, D. M. Identification of BRCA1-IRIS, a BRCA1 locus product. Nature Cell Biol. 6, 954–967 (2004).
Pettigrew, C. A. et al. Identification and functional analysis of novel BRCA1 transcripts, including mouse Brca1-Iris and human pseudo-BRCA1. Breast Cancer Res. Treat. 119, 239–247 (2010).
Chock, K. L., Allison, J. M., Shimizu, Y. & ElShamy, W. M. BRCA1-IRIS overexpression promotes cisplatin resistance in ovarian cancer cells. Cancer Res. 70, 8782–8791 (2010).
Ben David, Y. et al. Effect of BRCA mutations on the length of survival in epithelial ovarian tumors. J. Clin. Oncol. 20, 463–466 (2002).
Viguier, J. et al. ERCC1 codon 118 polymorphism is a predictive factor for the tumor response to oxaliplatin/5-fluorouracil combination chemotherapy in patients with advanced colorectal cancer. Clin. Cancer Res. 11, 6212–6217 (2005).
Zhou, W. et al. Excision repair cross-complementation group 1 polymorphism predicts overall survival in advanced non-small cell lung cancer patients treated with platinum-based chemotherapy. Clin. Cancer Res. 10, 4939–4943 (2004).
Zheng, H. et al. Nucleotide excision repair- and polymerase ε-mediated error-prone removal of mitomycin C interstrand cross-links. Mol. Cell. Biol. 23, 754–761 (2003).
Al-Minawi, A. Z. et al. The ERCC1/XPF endonuclease is required for completion of homologous recombination at DNA replication forks stalled by inter-strand cross-links. Nucleic Acids Res. 37, 6400–6413 (2009).
Olaussen, K. A. et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N. Engl. J. Med. 355, 983–991 (2006).
Postel-Vinay, S. et al. The potential of exploiting DNA-repair defects for optimizing lung cancer treatment. Nature Rev. Clin. Oncol. 9, 144–155 (2012).
Adamo, A. et al. Preventing nonhomologous end joining suppresses DNA repair defects of Fanconi anemia. Mol. Cell 39, 25–35 (2010).
Pace, P. et al. Ku70 corrupts DNA repair in the absence of the Fanconi anemia pathway. Science 329, 219–223 (2010).
Fattah, F. et al. Ku regulates the non-homologous end joining pathway choice of DNA double-strand break repair in human somatic cells. PLoS. Genet. 6, e1000855 (2010).
Bouwman, P. et al. 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nature Struct. Mol. Biol. 17, 688–695 (2010).
Bunting, S. F. et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell 141, 243–254 (2010).
Cao, L. et al. A selective requirement for 53BP1 in the biological response to genomic instability induced by Brca1 deficiency. Mol. Cell 35, 534–541 (2009). Together, references 111–113 show that loss of 53BP1 can partially restore HR in BRCA1-deficient cells and thereby decrease the response to therapy targeting HR deficiency.
Xie, A. et al. Distinct roles of chromatin-associated proteins MDC1 and 53BP1 in mammalian double-strand break repair. Mol. Cell 28, 1045–1057 (2007).
Pierce, A. J., Hu, P., Han, M., Ellis, N. & Jasin, M. Ku DNA end-binding protein modulates homologous repair of double-strand breaks in mammalian cells. Genes Dev. 15, 3237–3242 (2001).
Bennardo, N., Gunn, A., Cheng, A., Hasty, P. & Stark, J. M. Limiting the persistence of a chromosome break diminishes its mutagenic potential. PLoS. Genet. 5, e1000683 (2009).
DiTullio, R. A. Jr et al. 53BP1 functions in an ATM-dependent checkpoint pathway that is constitutively activated in human cancer. Nature Cell Biol. 4, 998–1002 (2002).
Fernandez-Capetillo, O. et al. DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nature Cell Biol. 4, 993–997 (2002).
Wang, B., Matsuoka, S., Carpenter, P. B. & Elledge, S. J. 53BP1, a mediator of the DNA damage checkpoint. Science 298, 1435–1438 (2002).
Oliver, T. G. et al. Chronic cisplatin treatment promotes enhanced damage repair and tumor progression in a mouse model of lung cancer. Genes Dev. 24, 837–852 (2010).
Sharma, S. V. et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 141, 69–80 (2010). This article shows that cancer cell subpopulations may undergo epigenetic alterations to withstand DNA-damaging chemotherapy.
Turner, N., Tutt, A. & Ashworth, A. Hallmarks of 'BRCAness' in sporadic cancers. Nature Rev. Cancer 4, 814–819 (2004).
Sorlie, T. et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl Acad. Sci. USA 98, 10869–10874 (2001).
van 't Veer, L. J. et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 415, 530–536 (2002).
Farmer, P. et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nature Med. 15, 68–74 (2009).
Borst, P. & Wessels, L. Do predictive signatures really predict response to cancer chemotherapy? Cell Cycle 9, 4836–4840 (2010).
Wessels, L. F. et al. Molecular classification of breast carcinomas by comparative genomic hybridization: a specific somatic genetic profile for BRCA1 tumors. Cancer Res. 62, 7110–7117 (2002).
Vollebergh, M. A. et al. An aCGH classifier derived from BRCA1-mutated breast cancer and benefit of high-dose platinum-based chemotherapy in HER2-negative breast cancer patients. Ann. Oncol. 22, 1561–1570 (2011).
Ionov, Y., Peinado, M. A., Malkhosyan, S., Shibata, D. & Perucho, M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 363, 558–561 (1993).
Esteller, M. et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N. Engl. J. Med. 343, 1350–1354 (2000).
Hegi, M. E. et al. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin. Cancer Res. 10, 1871–1874 (2004).
Graeser, M. K. et al. A marker of homologous recombination predicts pathological complete response to neoadjuvant chemotherapy in primary breast cancer. Clin. Cancer Res. 16, 6159–6168 (2010).
Bric, A. et al. Functional identification of tumor-suppressor genes through an in vivo RNA interference screen in a mouse lymphoma model. Cancer Cell 16, 324–335 (2009).
Meacham, C. E., Ho, E. E., Dubrovsky, E., Gertler, F. B. & Hemann, M. T. In vivo RNAi screening identifies regulators of actin dynamics as key determinants of lymphoma progression. Nature Genet. 41, 1133–1137 (2009).
Burgess, D. J. et al. Topoisomerase levels determine chemotherapy response in vitro and in vivo. Proc. Natl Acad. Sci. USA 105, 9053–9058 (2008).
Bryant, H. E. & Helleday, T. Inhibition of poly (ADP-ribose) polymerase activates ATM which is required for subsequent homologous recombination repair. Nucleic Acids Res. 34, 1685–1691 (2006).
Issaeva, N. et al. 6-thioguanine selectively kills BRCA2-defective tumors and overcomes PARP inhibitor resistance. Cancer Res. 70, 6268–6276 (2010).
Evers, B. et al. A high-throughput pharmaceutical screen identifies compounds with specific toxicity against BRCA2-deficient tumors. Clin. Cancer Res. 16, 99–108 (2010). In this article the authors combine a high-throughput pharmaceutical screen with genetically engineered mouse models to show that the bifunctional alkylators melphalan and nimustine may be more effective than platinum compounds or PARP inhibitors for the treatment of BRCA2-associated tumours, and thus may prevent the outgrowth of therapy-resistant tumours.
Osher, D. J., Kushner, Y. B., Arseneau, J. & Foulkes, W. D. Melphalan as a treatment for BRCA-related ovarian carcinoma: can you teach an old drug new tricks? J. Clin. Pathol. 64, 924–926 (2011).
Nieto, Y. & Shpall, E. J. High-dose chemotherapy for high-risk primary and metastatic breast cancer: is another look warranted? Curr. Opin. Oncol. 21, 150–157 (2009).
Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nature Rev. Cancer 7, 573–584 (2007).
Ledford, H. Drug candidates derailed in case of mistaken identity. Nature 483, 519 (2012).
Sequist, L. V. et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med. 3, 75ra26 (2011).
Sakai, W. et al. Functional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-mutated ovarian carcinoma. Cancer Res. 69, 6381–6386 (2009).
Chang, S., Biswas, K., Martin, B. K., Stauffer, S. & Sharan, S. K. Expression of human BRCA1 variants in mouse ES cells allows functional analysis of BRCA1 mutations. J. Clin. Invest. 119, 3160–3171 (2009).
Kuznetsov, S. G., Liu, P. & Sharan, S. K. Mouse embryonic stem cell-based functional assay to evaluate mutations in BRCA2. Nature Med. 14, 875–881 (2008).
Lee, M. S. et al. Comprehensive analysis of missense variations in the BRCT domain of BRCA1 by structural and functional assays. Cancer Res. 70, 4880–4890 (2010).
Liu, B. et al. Mismatch repair gene defects in sporadic colorectal cancers with microsatellite instability. Nature Genet. 9, 48–55 (1995).
Drost, M. et al. A rapid and cell-free assay to test the activity of lynch syndrome-associated MSH2 and MSH6 missense variants. Hum. Mutat. 33, 488–494 (2012).
Drost, M. et al. A cell-free assay for the functional analysis of variants of the mismatch repair protein MLH1. Hum. Mutat. 31, 247–253 (2010).
Acknowledgements
The authors are supported by grants from the Dutch Cancer Society, the Netherlands Organization of Scientific Research (NWO), the 7th framework programme of the European Union, and the Cancer Systems Biology Center (CSBC) funded by the NWO. We thank H. te Riele and S. Rottenberg for critically reading the Review, and M. van Vugt for helpful suggestions on figure 1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1
Overview of DDR proteins shown in Box 1 and their association with cancer (PDF 146 kb)
Related links
Glossary
- Homologous recombination
-
(HR). A recombination reaction between two homologous DNA duplexes.
- Synthetic lethal
-
A lethal combination of individually non-lethal mutations in two or more genes.
- Mitotic catastrophe
-
An event in which a cell is destroyed during mitosis because of aberrant chromosome segregation or DNA damage.
- Focus
-
DNA damage response foci are transiently formed sites in the nucleus where DNA damage response proteins are concentrated and recruited to DNA lesions.
- Intrinsic resistance
-
Primary (pre-existing) resistance to cytotoxic treatment.
- Acquired resistance
-
Secondary resistance, which results from natural selection of tumour cell clones carrying (epi)genetic alterations that confer resistance to cytotoxic treatment.
- Caretaker genes
-
Tumour suppressor genes that are required for the maintenance of genome stability.
- Missense mutations
-
Gene mutations that lead to the alteration of a single amino acid in the encoded protein.
- Hotspot mutations
-
Frequently occurring, nonrandom mutations.
- DNA end resection
-
The processing of DNA ends by nucleases to generate 3′ single-stranded DNA tails.
- Microsatellite instability
-
Variation in the length of microsatellites (repeating DNA sequences of up to 6 nucleotides).
- Hypomorphic mutations
-
Mutations that leave a gene partially functional.
Rights and permissions
About this article
Cite this article
Bouwman, P., Jonkers, J. The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat Rev Cancer 12, 587–598 (2012). https://doi.org/10.1038/nrc3342
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3342