Abstract
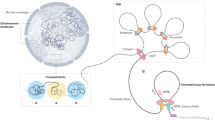
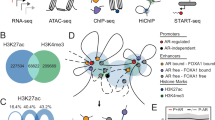
Pioneer factors are a special class of transcription factor that can associate with compacted chromatin to facilitate the binding of additional transcription factors. The function of pioneer factors was originally described during development; more recently, they have been implicated in hormone-dependent cancers, such as oestrogen receptor-positive breast cancer and androgen receptor-positive prostate cancer. We discuss the importance of pioneer factors in these specific cancers, the discovery of new putative pioneer factors and the interplay between these proteins in mediating nuclear receptor function in cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Zaret, K. S. & Carroll, J. S. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 25, 2227–2241 (2011).
Cirillo, L. A. & Zaret, K. S. An early developmental transcription factor complex that is more stable on nucleosome core particles than on free DNA. Mol. Cell 4, 961–969 (1999).
Sekiya, T. & Zaret, K. S. Repression by Groucho/TLE/Grg proteins: genomic site recruitment generates compacted chromatin in vitro and impairs activator binding in vivo. Mol. Cell 28, 291–303 (2007).
Cirillo, L. A. et al. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol. Cell 9, 279–289 (2002). References 2 and 4 are two important papers that showed that FOXA proteins can associate with compacted chromatin and can directly alter chromatin accessibility.
Grossmann, M. E., Huang, H. & Tindall, D. J. Androgen receptor signaling in androgen-refractory prostate cancer. J. Natl Cancer Inst. 93, 1687–1697 (2001).
Ali, S. & Coombes, R. C. Endocrine-responsive breast cancer and strategies for combating resistance. Nature Rev. Cancer 2, 101–112 (2002).
Kininis, M. et al. Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol. Cell Biol. 27, 5090–5104 (2007).
Cirillo, L. A. et al. Binding of the winged-helix transcription factor HNF3 to a linker histone site on the nucleosome. EMBO J. 17, 244–254 (1998).
Clark, K. L., Halay, E. D., Lai, E. & Burley, S. K. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature 364, 412–420 (1993).
Hah, N. et al. A rapid, extensive, and transient transcriptional response to estrogen signaling in breast cancer cells. Cell 145, 622–634 (2011).
Carroll, J. S. et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122, 33–43 (2005). This paper provided the first evidence that ER is co-bound by and requires the pioneer factor FOXA1.
Carroll, J. S. et al. Genome-wide analysis of estrogen receptor binding sites. Nature Genet. 38, 1289–1297 (2006).
Lin, C. Y. et al. Whole-genome cartography of estrogen receptor alpha binding sites. PLoS Genet. 3, e87 (2007).
Wang, Q. et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell 138, 245–256 (2009). This paper provided the first global map of AR binding in wild-type and drug-resistant cell lines, revealing important insight into AR-binding dynamics during progression to a castration-resistant phenotype.
Lupien, M. et al. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell 132, 958–970 (2008).
Hurtado, A., Holmes, K. A., Ross-Innes, C. S., Schmidt, D. & Carroll, J. S. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nature Genet. 43, 27–33 (2011).
Laganiere, J. et al. Location analysis of estrogen receptor alpha target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc. Natl Acad. Sci. USA 102, 11651–11656 (2005).
Wang, Q. et al. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol. Cell 27, 380–392 (2007).
Augello, M. A., Hickey, T. E. & Knudsen, K. E. FOXA1: master of steroid receptor function in cancer. EMBO J. 30, 3885–3894 (2011).
Wang, D. et al. Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature 474, 390–394 (2011).
Sahu, B. et al. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO J. 30, 3962–3976 (2011).
Yamaguchi, N. et al. FoxA1 as a lineage-specific oncogene in luminal type breast cancer. Biochem. Biophys. Res. Commun. 365, 711–717 (2008).
Zhang, C. et al. Definition of a FoxA1 Cistrome that is crucial for G1 to S-phase cell-cycle transit in castration-resistant prostate cancer. Cancer Res. 71, 6738–6748 (2011).
Lee, C. S., Friedman, J. R., Fulmer, J. T. & Kaestner, K. H. The initiation of liver development is dependent on Foxa transcription factors. Nature 435, 944–947 (2005).
Li, Z., Tuteja, G., Schug, J. & Kaestner, K. H. Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer. Cell 148, 72–83 (2012).
Holmes, K. A. et al. Transducin-like enhancer protein 1 mediates estrogen receptor binding and transcriptional activity in breast cancer cells. Proc. Natl Acad. Sci. USA 109, 2748–2753 (2012).
Tan, S. K. et al. AP-2gamma regulates oestrogen receptor-mediated long-range chromatin interaction and gene transcription. EMBO J. 30, 2569–2581 (2011).
Woodfield, G. W., Horan, A. D., Chen, Y. & Weigel, R. J. TFAP2C controls hormone response in breast cancer cells through multiple pathways of estrogen signaling. Cancer Res. 67, 8439–8443 (2007).
Magnani, L., Ballantyne, E. B., Zhang, X. & Lupien, M. PBX1 genomic pioneer function drives ERalpha signaling underlying progression in breast cancer. PLoS Genet. 7, e1002368 (2011). References 27 and 29 described two novel putative ER pioneer factors, AP2γ and PBX1. Both were shown to co-bind with ER and to be required for ER function.
Kouros-Mehr, H. et al. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell 13, 141–152 (2008).
Potter, A. S., Casa, A. J. & Lee, A. V. Forkhead box A1 (FOXA1) is a key mediator of insulin-like growth factor I (IGF-I) activity. J. Cell Biochem. 113, 110–121 (2012).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Orso, F. et al. Activator protein-2gamma (AP-2gamma) expression is specifically induced by oestrogens through binding of the oestrogen receptor to a canonical element within the 5'-untranslated region. Biochem. J. 377, 429–438 (2004).
Ross-Innes, C. S. et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 481, 389–393 (2012).
Serandour, A. A. et al. Epigenetic switch involved in activation of pioneer factor FOXA1-dependent enhancers. Genome Res. 21, 555–565 (2011).
Badve, S. et al. FOXA1 expression in breast cancer--correlation with luminal subtype A and survival. Clin. Cancer Res. 13, 4415–4421 (2007). This paper showed for the first time that a pioneer factor was prognostic in cancer patients.
Wolf, I. et al. FOXA1: Growth inhibitor and a favorable prognostic factor in human breast cancer. Int. J. Cancer 120, 1013–1022 (2007).
Jain, R. K., Mehta, R. J., Nakshatri, H., Idrees, M. T. & Badve, S. S. High-level expression of forkhead-box protein A1 in metastatic prostate cancer. Histopathology 58, 766–772 (2011).
Gee, J. M. et al. Overexpression of TFAP2C in invasive breast cancer correlates with a poorer response to anti-hormone therapy and reduced patient survival. J. Pathol. 217, 32–41 (2009).
Harrell, J. C. et al. Estrogen receptor positive breast cancer metastasis: altered hormonal sensitivity and tumor aggressiveness in lymphatic vessels and lymph nodes. Cancer Res. 66, 9308–9315 (2006).
Hegde, N. S., Sanders, D. A., Rodriguez, R. & Balasubramanian, S. The transcription factor FOXM1 is a cellular target of the natural product thiostrepton. Nat. Chem. 3, 829 (2011).
Shears, L., Plowright, L., Harrington, K., Pandha, H. S. & Morgan, R. Disrupting the interaction between HOX and PBX causes necrotic and apoptotic cell death in the renal cancer lines CaKi-2 and 769-P. J. Urol. 180, 2196–2201 (2008).
Morgan, R. et al. Antagonism of HOX/PBX dimer formation blocks the in vivo proliferation of melanoma. Cancer Res. 67, 5806–5813 (2007).
Kikugawa, T. et al. PLZF regulates Pbx1 transcription and Pbx1-HoxC8 complex leads to androgen-independent prostate cancer proliferation. Prostate 66, 1092–1099 (2006).
Sorlie, T. et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl Acad. Sci. USA 100, 8418–8423 (2003).
Acknowledgements
The authors thank G. Brown for help with data analysis and figures, and H. R. Ali for immunohistochemical images. The authors thank members of the Carroll laboratory and D. Odom for reading the manuscript. The authors would like to acknowledge the support of The University of Cambridge, Cancer Research UK and Hutchison Whampoa Limited.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Aromatase inhibitors
-
Therapies used to treat oestrogen receptor+ patients with breast cancer by decreasing circulating levels of oestrogenic compounds.
- Bicalutamide
-
An anti-androgen used for the treatment of prostate cancer.
- Luminal breast cancer
-
Oestrogen receptor+ breast cancer originating from the luminal cells.
- Pioneer factors
-
Proteins that physically interact with compacted chromatin, to rapidly facilitate the binding of additional proteins.
- Tamoxifen
-
Anti-oestrogen drug used for the treatment of oestrogen receptor (ER)+ patients with breast cancer. Tamoxifen binds to and blocks the ligand-binding pocket of ER.
Rights and permissions
About this article
Cite this article
Jozwik, K., Carroll, J. Pioneer factors in hormone-dependent cancers. Nat Rev Cancer 12, 381–385 (2012). https://doi.org/10.1038/nrc3263
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3263