Key Points
-
The serine–threonine liver kinase B1 (LKB1) is inactivated in Peutz–Jeghers syndrome and a large percentage of sporadic non-small cell lung carcinomas and cervical carcinomas.
-
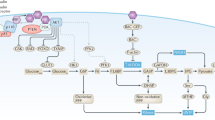
LKB1 acts a master upstream kinase, directly phosphorylating and activating AMP-activated protein kinase (AMPK) and a family of 12 related kinases that have crucial roles in cell growth, metabolism and polarity.
-
The LKB1–AMPK pathway serves as a metabolic checkpoint in the cell, arresting cell growth in conditions of low intracellular ATP levels, such as in low nutrient conditions.
-
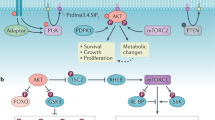
One of the central mitogenic pathways that is suppressed by LKB1 and AMPK signalling is the mTOR complex 1 pathway, which is inhibited through AMPK phosphorylation of tuberous sclerosis complex 2 and regulatory associated protein of mTOR (raptor).
-
Overnutrition and hyperglycaemia can suppress LKB1–AMPK signalling, which might contribute to an increased cancer risk in patients who are obese or diabetic. Conversely, activation of LKB1–AMPK signalling might contribute to the suppression of cancer risk that is associated with exercise and caloric restriction. Will AMPK-activating drugs, including existing diabetes therapeutics, find clinical usefulness as anticancer agents?
Abstract
In the past decade, studies of the human tumour suppressor LKB1 have uncovered a novel signalling pathway that links cell metabolism to growth control and cell polarity. LKB1 encodes a serine–threonine kinase that directly phosphorylates and activates AMPK, a central metabolic sensor. AMPK regulates lipid, cholesterol and glucose metabolism in specialized metabolic tissues, such as liver, muscle and adipose tissue. This function has made AMPK a key therapeutic target in patients with diabetes. The connection of AMPK with several tumour suppressors suggests that therapeutic manipulation of this pathway using established diabetes drugs warrants further investigation in patients with cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hong, S. P., Leiper, F. C., Woods, A., Carling, D. & Carlson, M. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc. Natl Acad. Sci. USA 100, 8839–8843 (2003).
Hawley, S. A. et al. Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2, 28 (2003).
Woods, A. et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 13, 2004–2008 (2003).
Shaw, R. J. et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl Acad. Sci. USA 101, 3329–3335 (2004).
Hemminki, A. et al. A serine/threonine kinase gene defective in Peutz–Jeghers syndrome. Nature 391, 184–187 (1998).
Sanchez-Cespedes, M. et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 62, 3659–3662 (2002).
Ji, H. et al. LKB1 modulates lung cancer differentiation and metastasis. Nature 448, 807–810 (2007). This paper describes the phenotype that results from the combined mutation of oncogenic Kras and LKB1 inactivation in a well-studied mouse model of KRAS-dependent lung carcinogenesis. LKB1 showed the most dramatic phenotype of any tumour suppressor tested when it was combined with Kras mutation.
Wingo, S. N. et al. Somatic LKB1 mutations promote cervical cancer progression. PLoS ONE 4, e5137 (2009).
Carling, D., Sanders, M. J. & Woods, A. The regulation of AMP-activated protein kinase by upstream kinases. Int. J. Obes. 32, S55–S59 (2008).
Lizcano, J. M. et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 23, 833–843 (2004).
Jaleel, M. et al. Identification of the sucrose non-fermenting related kinase SNRK, as a novel LKB1 substrate. FEBS Lett. 579, 1417–1423 (2005).
Al-Hakim, A. K. et al. 14-3-3 cooperates with LKB1 to regulate the activity and localization of QSK and SIK. J. Cell Sci. 118, 5661–5673 (2005).
Watts, J. L., Morton, D. G., Bestman, J. & Kemphues, K. J. The C. elegans par-4 gene encodes a putative serine–threonine kinase required for establishing embryonic asymmetry. Development 127, 1467–1475 (2000).
Anderson, K. A. et al. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 7, 377–388 (2008).
Tamas, P. et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J. Exp. Med. 203, 1665–1670 (2006).
Stahmann, N., Woods, A., Carling, D. & Heller, R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase-β. Mol. Cell. Biol. 26, 5933–5945 (2006).
Hawley, S. A. et al. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2, 9–19 (2005).
Woods, A. et al. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2, 21–33 (2005).
Hurley, R. L. et al. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 280, 29060–29066 (2005).
Hardie, D. G., Scott, J. W., Pan, D. A. & Hudson, E. R. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 546, 113–120 (2003).
Guertin, D. A. & Sabatini, D. M. Defining the role of mTOR in cancer. Cancer Cell 12, 9–22 (2007).
Wullschleger, S., Loewith, R. & Hall, M. N. TOR signaling in growth and metabolism. Cell 124, 471–484 (2006).
Holz, M. K., Ballif, B. A., Gygi, S. P. & Blenis, J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123, 569–80 (2005).
Choo, A. Y., Yoon, S. O., Kim, S. G., Roux, P. P. & Blenis, J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc. Natl Acad. Sci. USA 105, 17414–17419 (2008).
Thoreen, C. C. et al. An ATP-competitive mTOR inhibitor reveals rapamycin-insensitive functions of mTORC1. J. Biol. Chem. 284, 8023–8032 (2009).
Feldman, M. E. et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 7, e38 (2009).
Shaw, R. J. & Cantley, L. C. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 441, 424–430 (2006).
Huang, J. & Manning, B. D. The TSC1–TSC2 complex: a molecular switchboard controlling cell growth. Biochem. J. 412, 179–190 (2008).
Inoki, K., Zhu, T. & Guan, K. L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115, 577–590 (2003).
Corradetti, M. N., Inoki, K., Bardeesy, N., DePinho, R. A. & Guan, K. L. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz–Jeghers syndrome. Genes Dev. 18, 1533–1538 (2004).
Shaw, R. J. et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 6, 91–99 (2004).
Liu, L. et al. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol. Cell 21, 521–531 (2006).
Inoki, K. et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126, 955–968 (2006).
Hahn-Windgassen, A. et al. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J. Biol. Chem. 280, 32081–32089 (2005).
Gwinn, D. M. et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 30, 214–226 (2008). This study identified two highly conserved serines in the mTOR binding partner raptor as direct AMPK phosphorylation sites that are needed to inactivate mTORC1 signalling and promote cell cycle arrest.
Shackelford, D. B. et al. mTOR- and HIF-1α mediated tumor metabolism in an LKB1 mouse model of Peutz–Jeghers syndrome. Proc. Natl Acad. Sci. USA 18 Jun 2009 (doi:10.1073/pnas.0900465106).
Carretero, J. et al. Dysfunctional AMPK activity, signalling through mTOR and survival in response to energetic stress in LKB1-deficient lung cancer. Oncogene 26, 1616–1625 (2007).
Karuman, P. et al. The Peutz–Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol. Cell 7, 1307–1319 (2001).
Tiainen, M., Vaahtomeri, K., Ylikorkala, A. & Makela, T. P. Growth arrest by the LKB1 tumor suppressor: induction of p21WAF1/CIP1. Hum. Mol. Genet. 11, 1497–1504 (2002).
Imamura, K., Ogura, T., Kishimoto, A., Kaminishi, M. & Esumi, H. Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem. Biophys. Res. Commun. 287, 562–567 (2001).
Jones, R. G. et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell 18, 283–293 (2005).
Khanna, K. K. & Jackson, S. P. DNA double-strand breaks: signaling, repair and the cancer connection. Nature Genet. 27, 247–254 (2001).
Levine, A. J., Feng, Z., Mak, T. W., You, H. & Jin, S. Coordination and communication between the p53 and IGF-1–AKT–TOR signal transduction pathways. Genes Dev. 20, 267–275 (2006).
Budanov, A. V. & Karin, M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134, 451–460 (2008).
Feng, Z. et al. The regulation of AMPK β1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1–AKT–mTOR pathways. Cancer Res. 67, 3043–3053 (2007).
Greer, E. L. et al. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J. Biol. Chem. 282, 30107–30119 (2007).
Liang, J. et al. The energy sensing LKB1–AMPK pathway regulates p27kip1 phosphorylation mediating the decision to enter autophagy or apoptosis. Nature Cell Biol. 9, 218–224 (2007).
Short, J. D. et al. AMP-activated protein kinase signaling results in cytoplasmic sequestration of p27. Cancer Res. 68, 6496–6506 (2008).
Baba, M. et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc. Natl Acad. Sci. USA 103, 15552–15557 (2006).
Wang, W. et al. AMP-activated protein kinase-regulated phosphorylation and acetylation of importin α1: involvement in the nuclear import of RNA-binding protein HuR. J. Biol. Chem. 279, 48376–48388 (2004).
Carling, D., Zammit, V. A. & Hardie, D. G. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 223, 217–222 (1987).
Sato, R., Goldstein, J. L. & Brown, M. S. Replacement of serine-871 of hamster 3-hydroxy-3-methylglutaryl-CoA reductase prevents phosphorylation by AMP-activated kinase and blocks inhibition of sterol synthesis induced by ATP depletion. Proc. Natl Acad. Sci. USA 90, 9261–9265 (1993).
Zhan, Y. et al. Control of cell growth and survival by enzymes of the fatty acid synthesis pathway in HCT-116 colon cancer cells. Clin. Cancer Res. 14, 5735–5742 (2008).
Chajes, V., Cambot, M., Moreau, K., Lenoir, G. M. & Joulin, V. Acetyl-CoA carboxylase α is essential to breast cancer cell survival. Cancer Res. 66, 5287–5294 (2006).
Brusselmans, K., De Schrijver, E., Verhoeven, G. & Swinnen, J. V. RNA interference-mediated silencing of the acetyl-CoA-carboxylase-a gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Res. 65, 6719–6725 (2005).
Beckers, A. et al. Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res. 67, 8180–8187 (2007).
Orita, H. et al. Selective inhibition of fatty acid synthase for lung cancer treatment. Clin. Cancer Res. 13, 7139–7145 (2007).
Menendez, J. A. & Lupu, R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nature Rev. Cancer 7, 763–777 (2007).
Marsin, A. S. et al. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr. Biol. 10, 1247–1255 (2000).
Almeida, A., Moncada, S. & Bolanos, J. P. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nature Cell Biol. 6, 45–51 (2004).
Bando, H. et al. Phosphorylation of the 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase/PFKFB3 family of glycolytic regulators in human cancer. Clin. Cancer Res. 11, 5784–5792 (2005).
Telang, S. et al. Ras transformation requires metabolic control by 6-phosphofructo-2-kinase. Oncogene 25, 7225–7234 (2006).
Clem, B. et al. Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol. Cancer Ther 7, 110–120 (2008).
Yang, W. et al. Regulation of transcription by AMP-activated protein kinase: phosphorylation of p300 blocks its interaction with nuclear receptors. J. Biol. Chem. 276, 38341–38344 (2001).
Berdeaux, R. et al. SIK1 is a class II HDAC kinase that promotes survival of skeletal myocytes. Nature Med. 13, 597–603 (2007).
Dequiedt, F. et al. New role for hPar-1 kinases EMK and C-TAK1 in regulating localization and activity of class IIa histone deacetylases. Mol. Cell. Biol. 26, 7086–7102 (2006).
McGee, S. L. et al. AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes 57, 860–867 (2008).
Koo, S. H. et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437, 1109–1111 (2005).
Screaton, R. A. et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell 119, 61–74 (2004).
Jansson, D. et al. Glucose controls CREB activity in islet cells via regulated phosphorylation of TORC2. Proc. Natl Acad. Sci. USA 105, 10161–10166 (2008).
Shaw, R. J. et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 (2005). Using tissue-specific inactivation of LKB1 in mice, this study showed that LKB1-dependent signals are required in the liver for the widely used type 2 diabetes drug metformin to lower blood glucose.
Fu, A. & Screaton, R. A. Using kinomics to delineate signaling pathways: control of CRTC2/TORC2 by the AMPK family. Cell Cycle 7, 3823–3828 (2008).
Wu, L. et al. Transforming activity of MECT1–MAML2 fusion oncoprotein is mediated by constitutive CREB activation. EMBO J. 24, 2391–2402 (2005).
Canettieri, G. et al. The coactivator CRTC1 promotes cell proliferation and transformation via AP-1. Proc. Natl Acad. Sci. USA 106, 1445–1450 (2009).
Canto, C. et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–1060 (2009).
Jager, S., Handschin, C., St-Pierre, J. & Spiegelman, B. M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl Acad. Sci. USA 104, 12017–12022 (2007).
Brooks, C. L. & Gu, W. How does SIRT1 affect metabolism, senescence and cancer? Nature Rev. Cancer 9, 123–128 (2009).
Porstmann, T. et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 8, 224–236 (2008).
Zhou, G. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 (2001).
Kaelin, W. G. Jr & Ratcliffe, P. J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 (2008).
Shaw, R. J. Glucose metabolism and cancer. Curr. Opin. Cell Biol. 18, 598–608 (2006).
Denko, N. C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nature Rev. Cancer 8, 705–713 (2008).
Semenza, G. L. HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J. Bioenerg. Biomembr. 39, 231–234 (2007).
Majumder, P. K. et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nature Med. 10, 594–601 (2004).
Fantin, V. R., St-Pierre, J. & Leder, P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9, 425–434 (2006).
Brugarolas, J. et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 18, 2893–2904 (2004).
Martin, S. G. & St Johnston, D. A role for Drosophila LKB1 in anterior–posterior axis formation and epithelial polarity. Nature 421, 379–384 (2003).
Mirouse, V., Swick, L. L., Kazgan, N., St Johnston, D. & Brenman, J. E. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J. Cell Biol. 177, 387–392 (2007).
Lee, J. H. et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 447, 1017–1020 (2007).
Tomancak, P. et al. A Drosophila melanogaster homologue of Caenorhabditis elegans par-1 acts at an early step in embryonic-axis formation. Nature Cell Biol. 2, 458–460 (2000).
Shulman, J. M., Benton, R. & St Johnston, D. The Drosophila homolog of C. elegans PAR-1 organizes the oocyte cytoskeleton and directs oskar mRNA localization to the posterior pole. Cell 101, 377–388 (2000).
Baas, A. F. et al. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 116, 457–466 (2004). This study was the first to show a key role for mammalian LKB1 in establishing cell polarity, even in cells that lack cell–cell contacts.
Shelly, M., Cancedda, L., Heilshorn, S., Sumbre, G. & Poo, M. M. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell 129, 565–577 (2007).
Barnes, A. P. et al. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell 129, 549–563 (2007). References 93 and 94 show that LKB1 and its downstream SAD kinases play crucial parts in polarity and axonogenesis in the developing mammalian brain.
Hezel, A. F. & Bardeesy, N. LKB1; linking cell structure and tumor suppression. Oncogene 27, 6908–6919 (2008).
Kojima, Y. et al. Suppression of tubulin polymerization by the LKB1-microtubule-associated protein/microtubule affinity-regulating kinase signaling. J. Biol. Chem. 282, 23532–23540 (2007).
Biernat, J. et al. Protein kinase MARK/PAR-1 is required for neurite outgrowth and establishment of neuronal polarity. Mol. Biol. Cell 13, 4013–4028 (2002).
Sun, T. Q. et al. PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nature Cell Biol. 3, 628–636 (2001).
Ossipova, O., Dhawan, S., Sokol, S. & Green, J. B. Distinct PAR-1 proteins function in different branches of Wnt signaling during vertebrate development. Dev. Cell 8, 829–841 (2005).
Elbert, M., Cohen, D. & Musch, A. PAR1b promotes cell–cell adhesion and inhibits Dishevelled-mediated transformation of Madin-Darby canine kidney cells. Mol. Biol. Cell 17, 3345–3355 (2006).
Schlessinger, K., McManus, E. J. & Hall, A. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J. Cell Biol. 178, 355–361 (2007).
Zhang, X. et al. Dishevelled promotes axon differentiation by regulating atypical protein kinase C. Nature Cell Biol. 9, 743–754 (2007).
Narimatsu, M. et al. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell 137, 295–307 (2009).
Zhang, L., Li, J., Young, L. H. & Caplan, M. J. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc. Natl Acad. Sci. USA 103, 17272–17277 (2006).
Zheng, B. & Cantley, L. C. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc. Natl Acad. Sci. USA 104, 819–822 (2007).
Sebbagh, M., Santoni, M. J., Hall, B., Borg, J. P. & Schwartz, M. A. Regulation of LKB1/STRAD localization and function by E-cadherin. Curr. Biol. 19, 37–42 (2009).
Horman, S. et al. AMP-activated protein kinase phosphorylates and desensitizes smooth muscle myosin light chain kinase. J. Biol. Chem. 283, 18505–18512 (2008).
Yamamoto, H. et al. Identification of a novel substrate for TNFα-induced kinase NUAK2. Biochem. Biophys. Res. Commun. 365, 541–547 (2008).
ten Klooster, J. P. et al. Mst4 and Ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Dev. Cell 16, 551–562 (2009).
Partanen, J. I., Nieminen, A. I., Makela, T. P. & Klefstrom, J. Suppression of oncogenic properties of c-Myc by LKB1-controlled epithelial organization. Proc. Natl Acad. Sci. USA 104, 14694–14699 (2007).
Aranda, V. et al. Par6–aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nature Cell Biol. 8, 1235–1245 (2006).
Dow, L. E. et al. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene 26, 2272–2282 (2007).
Nolan, M. E. et al. The polarity protein Par6 induces cell proliferation and is overexpressed in breast cancer. Cancer Res. 68, 8201–8209 (2008).
Ylikorkala, A. et al. Vascular abnormalities and deregulation of VEGF in Lkb1-deficient mice. Science 293, 1323–1326 (2001).
Bardeesy, N. et al. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature 419, 162–167 (2002).
Miyoshi, H. et al. Gastrointestinal hamartomatous polyposis in Lkb1 heterozygous knockout mice. Cancer Res. 62, 2261–2266 (2002).
Jishage, K. et al. Role of Lkb1, the causative gene of Peutz–Jegher's syndrome, in embryogenesis and polyposis. Proc. Natl Acad. Sci. USA 99, 8903–8908 (2002).
Rossi, D. J. et al. Induction of cyclooxygenase-2 in a mouse model of Peutz–Jeghers polyposis. Proc. Natl Acad. Sci. USA 99, 12327–12332 (2002).
Katajisto, P. et al. LKB1 signaling in mesenchymal cells required for suppression of gastrointestinal polyposis. Nature Genet. 40, 455–459 (2008).
Vaahtomeri, K. et al. Lkb1 is required for TGFβ-mediated myofibroblast differentiation. J. Cell Sci. 121, 3531–3540 (2008).
Contreras, C. M. et al. Loss of Lkb1 provokes highly invasive endometrial adenocarcinomas. Cancer Res. 68, 759–766 (2008).
Carretero, J., Medina, P. P., Pio, R., Montuenga, L. M. & Sanchez-Cespedes, M. Novel and natural knockout lung cancer cell lines for the LKB1/STK11 tumor suppressor gene. Oncogene 23, 5084–5091 (2004).
Makowski, L. & Hayes, D. N. Role of LKB1 in lung cancer development. Br. J. Cancer 99, 683–688 (2008).
Gurumurthy, S., Hezel, A. F., Berger, J. H., Bosenberg, M. W. & Bardeesy, N. LKB1 deficiency sensitizes mice to carcinogen-induced tumorigenesis. Cancer Res. 68, 55–63 (2008).
Hardie, D. G. AMP-activated protein kinase as a drug target. Annu. Rev. Pharmacol. Toxicol. 47, 185–210 (2007).
Hundal, R. S. et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 49, 2063–2069 (2000).
Hardie, D. G. Neither LKB1 nor AMPK are the direct targets of metformin. Gastroenterology 131, 973 (2006).
Legro, R. S. et al. Ovulatory response to treatment of polycystic ovary syndrome is associated with a polymorphism in the STK11 gene. J. Clin. Endocrinol. Metab. 93, 792–800 (2008).
Shu, Y. et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J. Clin. Invest. 117, 1422–1431 (2007).
Schneider, M. B. et al. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology 120, 1263–1270 (2001).
Anisimov, V. N. et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp. Gerontol. 40, 685–693 (2005).
Zakikhani, M., Dowling, R., Fantus, I. G., Sonenberg, N. & Pollak, M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 66, 10269–10273 (2006).
Zakikhani, M., Dowling, R. J., Sonenberg, N. & Pollak, M. N. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev. Res. 1, 369–375 (2008).
Swinnen, J. V. et al. Mimicry of a cellular low energy status blocks tumor cell anabolism and suppresses the malignant phenotype. Cancer Res. 65, 2441–2448 (2005).
Buzzai, M. et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 67, 6745–6752 (2007). Following up these authors' previous finding that AMPK can activate a p53-dependent checkpoint, this study shows that metformin and AICAR have p53-dependent anti-tumour effects in xenografts.
Algire, C., Zakikhani, M., Blouin, M. J., Shuai, J. H. & Pollak, M. Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLC1 carcinoma growth. Endocr. Relat. Cancer 15, 833–839 (2008).
Huang, X. et al. Important role of the LKB1–AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem. J. 412, 211–221 (2008). This is the first study to directly examine the ability of metformin, phenformin and the targeted small molecule A769662, which activates AMPK, to suppress cancer in a spontaneously arising genetic mouse model.
Dykens, J. A. et al. Biguanide-induced mitochondrial dysfunction yields increased lactate production and cytotoxicity of aerobically-poised HepG2 cells and human hepatocytes in vitro. Toxicol. Appl. Pharmacol. 233, 203–210 (2008).
Owen, M. R., Doran, E. & Halestrap, A. P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 348, 607–614 (2000).
Scott, J. W. et al. Thienopyridone drugs are selective activators of AMP-activated protein kinase β1-containing complexes. Chem. Biol. 15, 1220–1230 (2008).
Cool, B. et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 3, 403–416 (2006).
Evans, J. M., Donnelly, L. A., Emslie-Smith, A. M., Alessi, D. R. & Morris, A. D. Metformin and reduced risk of cancer in diabetic patients. BMJ 330, 1304–1305 (2005).
Bowker, S. L., Majumdar, S. R., Veugelers, P. & Johnson, J. A. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 29, 254–258 (2006).
Jiralerspong, S. et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J. Clin. Oncol. 1 Jun 2009 (doi: 10.1200/JCO.2009.19.6410).
Goodwin, P. J., Ligibel, J. A. & Stambolic, V. Metformin in breast cancer: time for action. J. Clin. Oncol. 1 Jun 2009 (doi: 10.1200/JCO.2009.22.1630).
Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nature Rev. Cancer 8, 915–928 (2008).
Erdemoglu, E., Guney, M., Giray, S. G., Take, G. & Mungan, T. Effects of metformin on mammalian target of rapamycin in a mouse model of endometrial hyperplasia. Eur. J. Obstet. Gynecol. Reprod. Biol. 4 Jun 2009 (doi:10.1016/j.ejogrb.2009.04.034).
Memmott, R. M. et al. Phosphatidylinositol ether lipid analogues induce AMP-activated protein kinase-dependent death in LKB1-mutant non-small cell lung cancer cells. Cancer Res. 68, 580–588 (2008).
Nafz, J. et al. Interference with energy metabolism by 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside induces HPV suppression in cervical carcinoma cells and apoptosis in the absence of LKB1. Biochem. J. 403, 501–510 (2007).
Buzzai, M. et al. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid β-oxidation. Oncogene 21, 4165–4173 (2005).
Shell, S. A. et al. Activation of AMPK is necessary for killing cancer cells and sparing cardiac cells. Cell Cycle 7, 1769–1775 (2008).
Laderoute, K. R. et al. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol. Cell. Biol. 26, 5336–5347 (2006).
O'Connor, M. J., Martin, N. M. & Smith, G. C. Targeted cancer therapies based on the inhibition of DNA strand break repair. Oncogene 26, 7816–7824 (2007).
Podsypanina, K. et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/− mice. Proc. Natl Acad. Sci. USA 98, 10320–10325 (2001).
Johannessen, C. M. et al. TORC1 is essential for NF1-associated malignancies. Curr. Biol. 18, 56–62 (2008).
Lee, L. et al. Efficacy of a rapamycin analog (CCI-779) and IFN-γ in tuberous sclerosis mouse models. Genes Chromosom. Cancer 42, 213–227 (2005).
Wei, C. et al. Suppression of Peutz–Jeghers polyposis by targeting mammalian target of rapamycin signaling. Clin. Cancer Res. 14, 1167–1171 (2008).
Robinson, J. et al. Oral rapamycin reduces tumour burden and vascularization in Lkb1+/− mice. J. Pathol. 31 Mar 2009 (doi: 10.1002/path.2562).
Hudes, G. et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N. Engl. J. Med. 356, 2271–2281 (2007).
Cloughesy, T. F. et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 5, e8 (2008).
Bissler, J. J. et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N. Engl. J. Med. 358, 140–151 (2008).
Davies, D. M. et al. Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N. Engl. J. Med. 358, 200–203 (2008).
Martinez, M. E., Marshall, J. R. & Giovannucci, E. Diet and cancer prevention: the roles of observation and experimentation. Nature Rev. Cancer 8, 694–703 (2008).
McTiernan, A. Mechanisms linking physical activity with cancer. Nature Rev. Cancer 8, 205–211 (2008).
Jiang, W., Zhu, Z. & Thompson, H. J. Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Cancer Res. 68, 5492–5499 (2008).
Moore, T. et al. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev. Res. 1, 65–76 (2008).
Kelesidis, I., Kelesidis, T. & Mantzoros, C. S. Adiponectin and cancer: a systematic review. Br. J. Cancer 94, 1221–1225 (2006).
Vona-Davis, L., Howard-McNatt, M. & Rose, D. P. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes. Rev. 8, 395–408 (2007).
Sugiyama, M. et al. Adiponectin inhibits colorectal cancer cell growth through the AMPK/mTOR pathway. Int. J. Oncol. 34, 339–344 (2009).
Zheng, B. et al. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol. Cell 33, 237–247 (2009).
Hallstrom, T. C., Mori, S. & Nevins, J. R. An E2F1-dependent gene expression program that determines the balance between proliferation and cell death. Cancer Cell 13, 11–22 (2008).
Lee, M. & Vasioukhin, V. Cell polarity and cancer--cell and tissue polarity as a non-canonical tumor suppressor. J. Cell Sci. 121, 1141–1150 (2008).
Saadat, I. et al. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature 447, 330–333 (2007).
Hoyer-Hansen, M. & Jaattela, M. AMP-activated protein kinase: a universal regulator of autophagy? Autophagy 3, 381–383 (2007).
Wang, W., Yang, X., Lopez de Silanes, I., Carling, D. & Gorospe, M. Increased AMP:ATP ratio and AMP-activated protein kinase activity during cellular senescence linked to reduced HuR function. J. Biol. Chem. 278, 27016–27023 (2003).
Brugarolas, J. & Kaelin, W. G., Jr. Dysregulation of HIF and VEGF is a unifying feature of the familial hamartoma syndromes. Cancer Cell 6, 7–10 (2004).
Brugarolas, J. B., Vazquez, F., Reddy, A., Sellers, W. R. & Kaelin, W. G. Jr. TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell 4, 147–158 (2003).
Hurov, J. B., Watkins, J. L. & Piwnica-Worms, H. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr. Biol. 14, 736–741 (2004).
Suzuki, A. et al. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr. Biol. 14, 1425–1435 (2004).
Kusakabe, M. & Nishida, E. The polarity-inducing kinase Par-1 controls Xenopus gastrulation in cooperation with 14-3-3 and aPKC. EMBO J. 23, 4190–4201 (2004).
Zhang, Y. et al. PAR-1 kinase phosphorylates Dlg and regulates its postsynaptic targeting at the Drosophila neuromuscular junction. Neuron 53, 201–215 (2007).
Benton, R. & St Johnston, D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell 115, 691–704 (2003).
Ossipova, O., Bardeesy, N., DePinho, R. A. & Green, J. B. LKB1 (XEEK1) regulates Wnt signalling in vertebrate development. Nature Cell Biol. 5, 889–894 (2003).
Asada, N., Sanada, K. & Fukada, Y. LKB1 regulates neuronal migration and neuronal differentiation in the developing neocortex through centrosomal positioning. J. Neurosci 27, 11769–11775 (2007).
Zhang, S. et al. The tumor suppressor LKB1 regulates lung cancer cell polarity by mediating cdc42 recruitment and activity. Cancer Res. 68, 740–748 (2008).
Alessi, D. R., Sakamoto, K. & Bayascas, J. R. Lkb1-dependent signaling pathways. Annu. Rev. Biochem. 75, 137–163 (2006).
Puffenberger, E. G. et al. Polyhydramnios, megalencephaly and symptomatic epilepsy caused by a homozygous 7-kilobase deletion in LYK5. Brain 130, 1929–1941 (2007).
Towler, M. C. et al. A novel short splice variant of the tumour suppressor LKB1 is required for spermiogenesis. Biochem. J. 416, 1–14 (2008).
Denison, F. C., Hiscock, N. J., Carling, D. & Woods, A. Characterization of an alternative splice variant of LKB1. J. Biol. Chem. 284, 67–76 (2009).
Marignani, P. A. et al. Novel splice isoforms of STRADα differentially affect LKB1 activity, complex assembly and subcellular localization. Cancer Biol. Ther. 6, 1627–1631 (2007).
McBride, A., Ghilagaber, S., Nikolaev, A. & Hardie, D. G. The glycogen-binding domain on the AMPK β subunit allows the kinase to act as a glycogen sensor. Cell Metab. 9, 23–34 (2009).
Xiao, B. et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature 449, 496–500 (2007).
Sanders, M. J., Grondin, P. O., Hegarty, B. D., Snowden, M. A. & Carling, D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem. J. 403, 139–148 (2007).
Dolinsky, V. W. & Dyck, J. R. Role of AMP-activated protein kinase in healthy and diseased hearts. Am. J. Physiol. Heart Circ. Physiol. 291, H2557–H2569 (2006).
Robinson, J., Nye, E., Stamp, G. & Silver, A. Osteogenic tumours in Lkb1-deficient mice. Exp. Mol. Pathol. 85, 223–226 (2008).
Takeda, H., Miyoshi, H., Kojima, Y., Oshima, M. & Taketo, M. M. Accelerated onsets of gastric hamartomas and hepatic adenomas/carcinomas in Lkb1+/− p53−/− compound mutant mice. Oncogene 25, 1816–1820 (2006).
Wei, C. et al. Mutation of Lkb1 and p53 genes exert a cooperative effect on tumorigenesis. Cancer Res. 65, 11297–11303 (2005).
Shorning, B. Y. et al. Lkb1 deficiency alters goblet and paneth cell differentiation in the small intestine. PLoS ONE 4, e4264 (2009).
Pearson, H. B., McCarthy, A., Collins, C. M., Ashworth, A. & Clarke, A. R. Lkb1 deficiency causes prostate neoplasia in the mouse. Cancer Res. 68, 2223–2232 (2008).
Hezel, A. F. et al. Pancreatic LKB1 deletion leads to acinar polarity defects and cystic neoplasms. Mol. Cell. Biol. 28, 2414–2425 (2008).
Acknowledgements
We regret being unable to cite the work of many of our colleagues owing to space limitations. The authors thank K. Lamia for critical reading and editing of the manuscript. The authors' research is funded by grants from the National Institutes of Health (R01 DK080425 and P01 CA120964), American Cancer Society and V Foundation for Cancer Research to R.J.S. D.B.S. was supported by training grant T32 CA009370 to the Salk Institute Center for Cancer Research. R.J.S. is an early career scientist of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
National Cancer Institute Drug Dictionary
[18F] 2-fluoro-2-deoxy-D-glucose
FURTHER INFORMATION
Glossary
- Peutz–Jeghers syndrome
-
A disorder that is characterized by the development of gastrointestinal hamartomas and an increased predisposition to many other malignancies, including those arising in colon, breast, ovarian, pancreatic and lung tissues.
- Tuberous sclerosis complex
-
A familial tumour syndrome that is induced through mutation of the mTOR complex 1 regulators TSC1 and TSC2.
- Steatosis
-
Excess intracellular lipid accumulation, which can occur, for example, in the liver of patients who are diabetic or obese.
Rights and permissions
About this article
Cite this article
Shackelford, D., Shaw, R. The LKB1–AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer 9, 563–575 (2009). https://doi.org/10.1038/nrc2676
Issue Date:
DOI: https://doi.org/10.1038/nrc2676
This article is cited by
-
Caloric restriction and metformin selectively improved LKB1-mutated NSCLC tumor response to chemo- and chemo-immunotherapy
Journal of Experimental & Clinical Cancer Research (2024)
-
A high-content screen reveals new regulators of nuclear membrane stability
Scientific Reports (2024)
-
Glycolytic enzymes in non-glycolytic web: functional analysis of the key players
Cell Biochemistry and Biophysics (2024)
-
Paradoxical effects of statins on endothelial and cancer cells: the impact of concentrations
Cancer Cell International (2023)
-
Clinical prognosis and related molecular features of hepatitis B-associated adolescent and young adult hepatocellular carcinoma
Human Genomics (2023)