Key Points
-
Targeted protein proteolysis of key regulatory proteins by the ubiquitin–proteasome system (UPS) has a central role in maintaining and regulating growth. As such, components of the UPS can promote or prevent cellular transformation, which results from an aberrant response to otherwise normal cues that regulate processes involved in proliferation, differentiation and apoptosis.
-
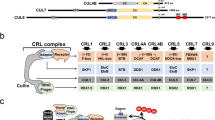
The SCF (SKP1–CUL1–F-box protein) ubiquitin ligases are the best characterized mammalian cullin RING ubiquitin ligases, and the F-box protein provides the substrate targeting specificity of the complex.
-
Out of sixty-nine F-box proteins that have been identified in humans, only nine have been matched with their respective substrates. The F-box proteins SKP2 (S-phase kinase-associated protein 2) and β-TrCP (β-transducin repeat-containing protein) have emerged as key regulatory molecules with roles in cellular processes that are intimately related to tumorigenesis.
-
SKP2 is an oncogenic protein that targets tumour suppressor proteins for degradation. As a positive regulator of cell cycle progression, a major target of SKP2 is the cyclin-dependent kinase (CDK) inhibitor p27, as has been shown in vivo and in vitro. Increased levels of SKP2 and reduced levels of p27 are observed in many types of cancer, and these levels are in several cases used as independent prognostic markers.
-
Whereas β-TrCP has been previously suggested to possess both oncogenic and tumour suppressive characteristics — mainly owing to the diversity in β-TrCP substrates — recent evidence indicates β-TrCP is mainly oncogenic.
-
Previous attempts at targeting components of the degradation machinery have been successful for laboratory and clinical use, as observed in the effectiveness of the proteasome inhibitor bortezomib (Velcade) in multiple myeloma. The development of pharmaceutical compounds targeting specific SCF ubiquitin ligases is timely and is complemented by structural and basic biochemical studies that have identified substrates for important cellular regulators such as SKP2 and β-TrCP.
Abstract
The maintenance and preservation of distinct phases during the cell cycle is a highly complex and coordinated process. It is regulated by phosphorylation — through the activity of cyclin-dependent kinases (CDKs) — and protein degradation, which occurs through ubiquitin ligases such as SCF (SKP1–CUL1–F-box protein) complexes and APC/C (anaphase-promoting complex/cyclosome). Here, we explore the functionality and biology of the F-box proteins, SKP2 (S-phase kinase-associated protein 2) and β-TrCP (β-transducin repeat-containing protein), which are emerging as important players in cancer biogenesis owing to the deregulated proteolysis of their substrates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hershko, A. The ubiquitin system for protein degradation and some of its roles in the control of the cell-division cycle (Nobel lecture). Angew. Chem. Int. Ed. Engl. 44, 5932–5943 (2005). An historical perspective about the discovery of the ubiquitin system that describes how E1, E2 and E3 enzymes work together to promote ubiquitin ligation to substrates.
Petroski, M. D. & Deshaies, R. J. Function and regulation of cullin-RING ubiquitin ligases. Nature Rev. Mol. Cell Biol. 6, 9–20 (2005). An excellent review of cullin RING ubiquitin ligases.
Cardozo, T. & Pagano, M. The SCF ubiquitin ligase: insights into a molecular machine. Nature Rev. Mol. Cell Biol. 5, 739–751 (2004).
Jin, J. et al. Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev. 18, 2573–2580 (2004).
Cenciarelli, C. et al. Identification of a family of human F-box proteins. Curr. Biol. 9, 1177–1179 (1999).
Winston, J. T., Koepp, D. M., Zhu, C., Elledge, S. J. & Harper, J. W. A family of mammalian F-box proteins. Curr. Biol. 9, 1180–1182 (1999). References 4–6 classify the mammalian family of F-box proteins.
Welcker, M. & Clurman, B. E. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nature Rev. Cancer 8, 83–93 (2007). An excellent and up-to-date review about FBXW7 and its role in cancer.
Malumbres, M. & Barbacid, M. Cell cycle kinases in cancer. Curr. Opin. Genet. Dev. 17, 60–65 (2007).
Guardavaccaro, D. & Pagano, M. Stabilizers and destabilizers controlling cell cycle oscillators. Mol. Cell 22, 1–4 (2006).
Branzei, D. & Foiani, M. Regulation of DNA repair throughout the cell cycle. Nature Rev. Mol. Cell Biol. 9, 297–308 (2008).
Zhang, H., Kobayashi, R., Galaktionov, K. & Beach, D. p19Skp1 and p45Skp2 are essential elements of the cyclin A–CDK2 S phase kinase. Cell 82, 915–925 (1995).
Carrano, A. C., Eytan, E., Hershko, A. & Pagano, M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nature Cell Biol. 1, 193–199 (1999).
Sutterluty, H. et al. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nature Cell Biol. 1, 207–214 (1999).
Tsvetkov, L. M., Yeh, K. H., Lee, S. J., Sun, H. & Zhang, H. p27Kip1 ubiquitination and degradation is regulated by the SCFSkp2 complex through phosphorylated Thr187 in p27. Curr. Biol. 9, 661–664 (1999). References 12–14 characterize the function of SKP2 in cell cycle control and the ubiquitin-mediated degradation of the tumour suppressor p27.
Spruck, C. et al. A CDK-independent function of mammalian Cks1: targeting of SCFSkp2 to the CDK inhibitor p27Kip1. Mol. Cell 7, 639–650 (2001).
Ganoth, D. et al. The cell-cycle regulatory protein Cks1 is required for SCFSkp2-mediated ubiquitinylation of p27. Nature Cell Biol. 3, 321–324 (2001).
Bloom, J. & Pagano, M. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin. Cancer Biol. 13, 41–47 (2003).
Nakayama, K. et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27Kip1, polyploidy and centrosome overduplication. EMBO J. 19, 2069–2081 (2000). Shows that deletion of SKP2 results in accumulation of p27 in vivo.
Nakayama, K. et al. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev. Cell 6, 661–672 (2004). Shows that p27 loss reverts most of the phenotypes that are due to SKP2-deficiency and that the SKP2–p27 axis functions not only at G1–S, but also at G2–M.
Kossatz, U. et al. Skp2-dependent degradation of p27kip1 is essential for cell cycle progression. Genes Dev. 18, 2602–2607 (2004).
Bornstein, G. et al. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S. phase. J. Biol. Chem. 278, 25752–25757 (2003).
Yu, Z. K., Gervais, J. L. & Zhang, H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21CIP1/WAF1 and cyclin D proteins. Proc. Natl Acad. Sci. USA 95, 11324–11329 (1998). The first evidence that SKP2 targets p21, a tumour suppressor protein, for degradation.
Kamura, T. et al. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc. Natl Acad. Sci. USA 100, 10231–10236 (2003).
Hiramatsu, Y. et al. Degradation of Tob1 mediated by SCFSkp2-dependent ubiquitination. Cancer Res. 66, 8477–8483 (2006).
Song, M. S. et al. Skp2 regulates the antiproliferative function of the tumor suppressor RASSF1A via ubiquitin-mediated degradation at the G(1)–S transition. Oncogene 10 Dec 2007 (doi:10.1038/sj.onc.1210971).
Tedesco, D., Lukas, J. & Reed, S. I. The pRb-related protein p130 is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCFSkp2. Genes Dev. 16, 2946–2957 (2002).
Huang, H. et al. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc. Natl Acad. Sci. USA 102, 1649–1654 (2005). Identifies FOXO1 as a substrate of SKP2 and suggests SKP2-promoted proteolysis might have a role in tumorigenesis.
Tokarz, S. et al. The ISG15 isopeptidase UBP43 is regulated by proteolysis via the SCFSkp2 ubiquitin ligase. J. Biol. Chem. 279, 46424–46430 (2004).
Garriga, J. et al. CDK9 is constitutively expressed throughout the cell cycle, and its steady-state expression is independent of SKP2. Mol. Cell. Biol. 23, 5165–5173 (2003).
Kiernan, R. E. et al. Interaction between cyclin T1 and SCFSKP2 targets CDK9 for ubiquitination and degradation by the proteasome. Mol. Cell. Biol. 21, 7956–7970 (2001).
Carrano, A. C. & Pagano, M. Role of the F-box protein Skp2 in adhesion-dependent cell cycle progression. J. Cell Biol. 153, 1381–1390 (2001).
Signoretti, S. et al. Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J. Clin. Invest. 110, 633–641 (2002).
Waltregny, D. et al. Androgen-driven prostate epithelial cell proliferation and differentiation in vivo involve the regulation of p27. Mol. Endocrinol. 15, 765–782 (2001).
Lu, L., Schulz, H. & Wolf, D. A. The F-box protein SKP2 mediates androgen control of p27 stability in LNCaP human prostate cancer cells. BMC Cell Biol. 3, 22 (2002).
Latres, E. et al. Role of the F-box protein Skp2 in lymphomagenesis. Proc. Natl Acad. Sci. USA 98, 2515–2520 (2001).
Kang-Decker, N. et al. Loss of CBP causes T cell lymphomagenesis in synergy with p27Kip1 insufficiency. Cancer Cell 5, 177–189 (2004).
Shim, E. H. et al. Expression of the F-box protein SKP2 induces hyperplasia, dysplasia, and low-grade carcinoma in the mouse prostate. Cancer Res. 63, 1583–1588 (2003).
Radke, S., Pirkmaier, A. & Germain, D. Differential expression of the F-box proteins Skp2 and Skp2B in breast cancer. Oncogene 24, 3448–3458 (2005).
Timmerbeul, I. et al. Testing the importance of p27 degradation by the SCFskp2 pathway in murine models of lung and colon cancer. Proc. Natl Acad. Sci. USA 103, 14009–14014 (2006).
Keller, U. B. et al. Myc targets Cks1 to provoke the suppression of p27Kip1, proliferation and lymphomagenesis. EMBO J. 26, 2562–2574 (2007).
Philipp-Staheli, J., Payne, S. R. & Kemp, C. J. p27Kip1: regulation and function of a haploinsufficient tumor suppressor and its misregulation in cancer. Exp. Cell Res. 264, 148–168 (2001).
Slotky, M. et al. The expression of the ubiquitin ligase subunit Cks1 in human breast cancer. Breast Cancer Res. 7, R737–R744 (2005).
Shapira, M. et al. The prognostic impact of the ubiquitin ligase subunits Skp2 and Cks1 in colorectal carcinoma. Cancer 103, 1336–1346 (2005).
Shapira, M. et al. Alterations in the expression of the cell cycle regulatory protein cyclin kinase subunit 1 in colorectal carcinoma. Cancer 100, 1615–1621 (2004).
Masuda, T. A. et al. Cyclin-dependent kinase 1 gene expression is associated with poor prognosis in gastric carcinoma. Clin. Cancer Res. 9, 5693–5698 (2003).
Hershko, D. D. & Shapira, M. Prognostic role of p27Kip1 deregulation in colorectal cancer. Cancer 107, 668–675 (2006).
Kamura, T. et al. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27Kip1 at G1 phase. Nature Cell Biol. 6, 1229–1235 (2004).
Hattori, T. et al. Pirh2 promotes ubiquitin-dependent degradation of the cyclin-dependent kinase inhibitor p27Kip1. Cancer Res. 67, 10789–10795 (2007).
Goto, T. et al. Mechanism and functional consequences of loss of FOXO1 expression in endometrioid endometrial cancer cells. Oncogene 27, 9–19 (2008).
Bellan, C. et al. Missing expression of pRb2/p130 in human retinoblastomas is associated with reduced apoptosis and lesser differentiation. Invest. Ophthalmol. Vis. Sci. 43, 3602–3608 (2002).
Caputi, M. et al. Loss of pRb2/p130 expression is associated with unfavorable clinical outcome in lung cancer. Clin. Cancer Res. 8, 3850–3856 (2002).
D'Andrilli, G. et al. Frequent loss of pRb2/p130 in human ovarian carcinoma. Clin. Cancer Res. 10, 3098–3103 (2004).
Helin, K. et al. Loss of the retinoblastoma protein-related p130 protein in small cell lung carcinoma. Proc. Natl Acad. Sci. USA 94, 6933–6938 (1997).
Scambia, G., Lovergine, S. & Masciullo, V. RB family members as predictive and prognostic factors in human cancer. Oncogene 25, 5302–5308 (2006).
Susini, T. et al. Expression of the retinoblastoma-related gene Rb2/p130 correlates with clinical outcome in endometrial cancer. J. Clin. Oncol. 16, 1085–1093 (1998).
Zamparelli, A. et al. Expression of cell-cycle-associated proteins pRB2/p130 and p27kip in vulvar squamous cell carcinomas. Hum. Pathol. 32, 4–9 (2001).
Soldatenkov, V. A., Dritschilo, A., Ronai, Z. & Fuchs, S. Y. Inhibition of homologue of Slimb (HOS) function sensitizes human melanoma cells for apoptosis. Cancer Res. 59, 5085–5088 (1999).
Busino, L. et al. Degradation of Cdc25A by β-TrCP during S phase and in response to DNA damage. Nature 426, 87–91 (2003).
Tang, W. et al. Targeting β-transducin repeat-containing protein E3 ubiquitin ligase augments the effects of antitumor drugs on breast cancer cells. Cancer Res. 65, 1904–1908 (2005).
Guardavaccaro, D. et al. Control of meiotic and mitotic progression by the F box protein β-Trcp1 in vivo. Dev. Cell 4, 799–812 (2003).
Nakayama, K. et al. Impaired degradation of inhibitory subunit of NF-κB (IκB) and β-catenin as a result of targeted disruption of the β-TrCP1 gene. Proc. Natl Acad. Sci. USA 100, 8752–8757 (2003).
Mailand, N., Bekker-Jensen, S., Bartek, J. & Lukas, J. Destruction of Claspin by SCFβTrCP restrains Chk1 activation and facilitates recovery from genotoxic stress. Mol. Cell 23, 307–318 (2006).
Peschiaroli, A. et al. SCFβTrCP-mediated degradation of Claspin regulates recovery from the DNA replication checkpoint response. Mol. Cell 23, 319–329 (2006).
Watanabe, N. et al. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFβ-TrCP. Proc. Natl Acad. Sci. USA 101, 4419–4424 (2004).
Ougolkov, A. et al. Associations among β-TrCP, an E3 ubiquitin ligase receptor, β-catenin, and NF-κB in colorectal cancer. J. Natl Cancer Inst. 96, 1161–1170 (2004).
Muerkoster, S. et al. Increased expression of the E3-ubiquitin ligase receptor subunit βTRCP1 relates to constitutive nuclear factor-κB activation and chemoresistance in pancreatic carcinoma cells. Cancer Res. 65, 1316–1324 (2005).
Koch, A. et al. Elevated expression of Wnt antagonists is a common event in hepatoblastomas. Clin. Cancer Res. 11, 4295–4304 (2005).
Spiegelman, V. S. et al. Induction of homologue of Slimb ubiquitin ligase receptor by mitogen signaling. J. Biol. Chem. 277, 36624–36630 (2002).
Kudo, Y. et al. Role of F-box protein βTrcp1 in mammary gland development and tumorigenesis. Mol. Cell. Biol. 24, 8184–8194 (2004). Shows that β-TrCP1 positively controls the proliferation of breast epithelium and its overexpression induces transformation in the breast epithelium.
Karin, M. & Greten, F. R. NF-κB: linking inflammation and immunity to cancer development and progression. Nature Rev. Immunol. 5, 749–759 (2005).
Wu, C. & Ghosh, S. β-TrCP mediates the signal-induced ubiquitination of IκBβ. J. Biol. Chem. 274, 29591–29594 (1999).
Shirane, M., Hatakeyama, S., Hattori, K., Nakayama, K. & Nakayama, K. Common pathway for the ubiquitination of IκBα, IκBβ, and IκBε mediated by the F-box protein FWD1. J. Biol. Chem. 274, 28169–28174 (1999).
Tan, P. et al. Recruitment of a ROC1-CUL1 ubiquitin ligase by Skp1 and HOS to catalyze the ubiquitination of IκBα. Mol. Cell 3, 527–533 (1999). One of the first papers showing that the SCF contains the RING-finger protein RBX1 and that an SCF containing β-TrCP targets IκBα for degradation.
Kroll, M. et al. Inducible degradation of IkBa by the proteasome requires interaction with the F-box protein h-βTrCP. J. Biol. Chem. 274, 7941–7945 (1999).
Spencer, E., Jiang, J. & Chen, Z. J. Signal-induced ubiquitination of IκBα by the F-box protein Slimb/β-TrCP. Genes Dev. 13, 284–294 (1999).
Winston, J. T. et al. The SCFβ-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 13, 270–283 (1999).
Yaron, A. et al. Identification of the receptor component of the IκBα-ubiquitin ligase. Nature 396, 590–594 (1998).
Hatakeyama, S. et al. Ubiquitin-dependent degradation of IκBα a is mediated by a ubiquitin ligase Skp1/Cul1/F-box protein FWD1. Proc. Natl Acad. Sci. USA 96, 3859–3863 (1999).
Arsura, M. & Cavin, L. G. Nuclear factor-κB and liver carcinogenesis. Cancer Lett. 229, 157–169 (2005).
Pikarsky, E. et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 431, 461–466 (2004).
Dhawan, P. & Richmond, A. A novel NF-κB-inducing kinase-MAPK signaling pathway up-regulates NF-κB activity in melanoma cells. J. Biol. Chem. 277, 7920–7928 (2002).
Liu, J. et al. Oncogenic BRAF regulates β-Trcp expression and NF-κB activity in human melanoma cells. Oncogene 26, 1954–1958 (2007).
Yang, H. S. et al. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol. Cell. Biol. 23, 26–37 (2003).
Dorrello, N. V. et al. S6K1- and βTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science 314, 467–471 (2006).
Afonja, O., Juste, D., Das, S., Matsuhashi, S. & Samuels, H. H. Induction of PDCD4 tumor suppressor gene expression by RAR agonists, antiestrogen and HER-2/neu antagonist in breast cancer cells. Evidence for a role in apoptosis. Oncogene 23, 8135–8145 (2004).
Goke, R., Barth, P., Schmidt, A., Samans, B. & Lankat-Buttgereit, B. Programmed cell death protein 4 suppresses CDK1/cdc2 via induction of p21Waf1/Cip1. Am. J. Physiol. Cell Physiol. 287, C1541–C1546 (2004).
Wen, Y. H. et al. Alterations in the expression of PDCD4 in ductal carcinoma of the breast. Oncol. Rep. 18, 1387–1393 (2007).
Zhang, H. et al. Involvement of programmed cell death 4 in transforming growth factor-β1-induced apoptosis in human hepatocellular carcinoma. Oncogene 25, 6101–6112 (2006).
Mudduluru, G. et al. Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer 110, 1697–1707 (2007).
Chen, Y. et al. Loss of PDCD4 expression in human lung cancer correlates with tumour progression and prognosis. J. Pathol. 200, 640–646 (2003).
Majumder, S. REST in good times and bad: roles in tumor suppressor and oncogenic activities. Cell Cycle 5, 1929–1935 (2006).
Westbrook, T. F. et al. A genetic screen for candidate tumor suppressors identifies REST. Cell 121, 837–848 (2005).
Westbrook, T. F. et al. SCFβ-TRCP controls oncogenic transformation and neural differentiation through REST degradation. Nature 452, 370–374 (2008).
Saitoh, T. & Katoh, M. Expression profiles of βTRCP1 and βTRCP2, and mutation analysis of βTRCP2 in gastric cancer. Int. J. Oncol. 18, 959–964 (2001).
Kim, C. J. et al. Somatic mutations of the β-TrCP gene in gastric cancer. Apmis 115, 127–133 (2007).
Gerstein, A. V. et al. APC/CTNNB1 (β-catenin) pathway alterations in human prostate cancers. Genes Chromosomes Cancer 34, 9–16 (2002).
Wood, L. D. et al. The genomic landscapes of human breast and colorectal cancers. Science 318, 1108–1113 (2007).
Clevers, H. Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 (2006).
Liu, C. et al. β-Trcp couples β-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc. Natl Acad. Sci. USA 96, 6273–6278 (1999).
Kitagawa, M. et al. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of β-catenin. EMBO J. 18, 2401–2410 (1999).
Lagna, G., Carnevali, F., Marchioni, M. & Hemmati-Brivanlou, A. Negative regulation of axis formation and Wnt signaling in Xenopus embryos by the F-box/WD40 protein βTrCP. Mech. Dev. 80, 101–106 (1999).
Hart, M. et al. The F-box protein β-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Curr. Biol. 9, 207–210 (1999).
Latres, E., Chiaur, D. S. & Pagano, M. The human F box protein β-Trcp associates with the Cul1/Skp1 complex and regulates the stability of β-catenin. Oncogene 18, 849–854 (1999).
Marikawa, Y. & Elinson, R. P. β-TrCP is a negative regulator of Wnt/β-catenin signaling pathway and dorsal axis formation in Xenopus embryos. Mech. Dev. 77, 75–80 (1998).
Li, Y. et al. Stabilization of prolactin receptor in breast cancer cells. Oncogene 25, 1896–1902 (2006).
Guardavaccaro, D. et al. Control of chromosome stability by the β-TrCP–REST–Mad2 axis. Nature 452, 365–369 (2008).
Fuller, G. N. et al. Many human medulloblastoma tumors overexpress repressor element-1 silencing transcription (REST)/neuron-restrictive silencer factor, which can be functionally countered by REST-VP16. Mol. Cancer Ther. 4, 343–349 (2005).
Su, X., Kameoka, S., Lentz, S. & Majumder, S. Activation of REST/NRSF target genes in neural stem cells is sufficient to cause neuronal differentiation. Mol. Cell. Biol. 24, 8018–8025 (2004).
Lawinger, P. et al. The neuronal repressor REST/NRSF is an essential regulator in medulloblastoma cells. Nature Med. 6, 826–831 (2000).
Kanemori, Y., Uto, K. & Sagata, N. β-TrCP recognizes a previously undescribed nonphosphorylated destruction motif in Cdc25A and Cdc25B phosphatases. Proc. Natl Acad. Sci. USA 102, 6279–6284 (2005).
Boutros, R., Lobjois, V. & Ducommun, B. CDC25 phosphatases in cancer cells: key players? Good targets? Nature Rev. Cancer 7, 495–507 (2007).
Cangi, M. G. et al. Role of the Cdc25A phosphatase in human breast cancer. J. Clin. Invest. 106, 753–761 (2000).
Kristjansdottir, K. & Rudolph, J. Cdc25 phosphatases and cancer. Chem. Biol. 11, 1043–1051 (2004).
Hernandez, S. et al. Cdc25 cell cycle-activating phosphatases and c-myc expression in human non-Hodgkin's lymphomas. Cancer Res. 58, 1762–1767 (1998).
Hernandez, S. et al. Cdc25a and the splicing variant cdc25b2, but not cdc25B1, -B3 or -C, are over-expressed in aggressive human non-Hodgkin's lymphomas. Int. J. Cancer 89, 148–152 (2000).
Ito, Y. et al. Cdc25A and cdc25B expression in malignant lymphoma of the thyroid: correlation with histological subtypes and cell proliferation. Int. J. Mol. Med. 13, 431–435 (2004).
Loffler, H. et al. Distinct modes of deregulation of the proto-oncogenic Cdc25A phosphatase in human breast cancer cell lines. Oncogene 22, 8063–8071 (2003).
Hsu, J. Y., Reimann, J. D., Sorensen, C. S., Lukas, J. & Jackson, P. K. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APCCdh1. Nature Cell Biol. 4, 358–366 (2002).
Gutgemann, I., Lehman, N. L., Jackson, P. K. & Longacre, T. A. Emi1 protein accumulation implicates misregulation of the anaphase promoting complex/cyclosome pathway in ovarian clear cell carcinoma. Mod. Pathol. 21, 445–454 (2008).
Lehman, N. L. et al. Oncogenic regulators and substrates of the anaphase promoting complex/cyclosome are frequently overexpressed in malignant tumors. Am. J. Pathol. 170, 1793–1805 (2007).
Adams, J. & Kauffman, M. Development of the proteasome inhibitor Velcade (Bortezomib). Cancer Invest. 22, 304–311 (2004).
Busino, L. et al. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science 316, 900–904 (2007).
Gallego, M. & Virshup, D. M. Post-translational modifications regulate the ticking of the circadian clock. Nature Rev. Mol. Cell Biol. 8, 139–148 (2007).
Frescas, D., Guardavaccaro, D., Bassermann, F., Koyama-Nasu, R. & Pagano, M. JHDM1B/FBXL10 is a nucleolar protein that represses transcription of ribosomal RNA genes. Nature 450, 309–313 (2007).
Bassermann, F. et al. NIPA defines an SCF-type mammalian E3 ligase that regulates mitotic entry. Cell 122, 45–57 (2005).
Amador, V., Ge, S., Santamaria, P. G., Guardavaccaro, D. & Pagano, M. APC/CCdc20 controls the ubiquitin-mediated degradation of p21 in prometaphase. Mol. Cell 27, 462–473 (2007).
Li, X., Zhao, Q., Liao, R., Sun, P. & Wu, X. The SCFSkp2 ubiquitin ligase complex interacts with the human replication licensing factor Cdt1 and regulates Cdt1 degradation. J. Biol. Chem. 278, 30854–30858 (2003).
Mendez, J. et al. Human origin recognition complex large subunit is degraded by ubiquitin-mediated proteolysis after initiation of DNA replication. Mol. Cell 9, 481–491 (2002).
Moro, L., Arbini, A. A., Marra, E. & Greco, M. Up-regulation of Skp2 after prostate cancer cell adhesion to basement membranes results in BRCA2 degradation and cell proliferation. J. Biol. Chem. 281, 22100–22107 (2006).
Jiang, H. et al. Ubiquitylation of RAG-2 by Skp2-SCF links destruction of the V(D)J. recombinase to the cell cycle. Mol. Cell 18, 699–709 (2005).
Liu, Y. et al. The ETS protein MEF is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCFSkp2. Mol. Cell. Biol. 26, 3114–3123 (2006).
Liu, H., Cheng, E. H. & Hsieh, J. J. Bimodal degradation of MLL by SCFSkp2 and APCCdc20 assures cell cycle execution: a critical regulatory circuit lost in leukemogenic MLL fusions. Genes Dev. 21, 2385–2398 (2007).
Charrasse, S., Carena, I., Brondani, V., Klempnauer, K. H. & Ferrari, S. Degradation of B-Myb by ubiquitin-mediated proteolysis: involvement of the Cdc34-SCFp45Skp2 pathway. Oncogene 19, 2986–2995 (2000).
von der Lehr, N., Johansson, S. & Larsson, L. G. Implication of the ubiquitin/proteasome system in Myc-regulated transcription. Cell Cycle 2, 403–407 (2003).
von der Lehr, N. et al. The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol. Cell 11, 1189–1200 (2003).
Marti, A., Wirbelauer, C., Scheffner, M. & Krek, W. Interaction between ubiquitin-protein ligase SCFSKP2 and E2F-1 underlies the regulation of E2F-1 degradation. Nature Cell Biol. 1, 14–19 (1999).
Oh, K. J. et al. The papillomavirus E7 oncoprotein is ubiquitinated by UbcH7 and Cullin 1- and Skp2-containing E3 ligase. J. Virol. 78, 5338–5346 (2004).
Lin, Y. W. & Yang, J. L. Cooperation of ERK and SCFSkp2 for MKP-1 destruction provides a positive feedback regulation of proliferating signaling. J. Biol. Chem. 281, 915–926 (2006).
Liang, M. et al. Ubiquitination and proteolysis of cancer-derived Smad4 mutants by SCFSkp2. Mol. Cell. Biol. 24, 7524–7537 (2004).
Huang, Z., Nie, L., Xu, M. & Sun, X. H. Notch-induced E2A degradation requires CHIP and Hsc70 as novel facilitators of ubiquitination. Mol. Cell. Biol. 24, 8951–8962 (2004).
Nie, L., Xu, M., Vladimirova, A. & Sun, X. H. Notch-induced E2A ubiquitination and degradation are controlled by MAP kinase activities. EMBO J. 22, 5780–5792 (2003).
Nie, L., Wu, H. & Sun, X. H. Ubiquitination and degradation of Tal1/SCL is induced by Notch signaling and depends on Skp2 and CHIP. J. Biol. Chem. (2007).
Sanada, T. et al. Skp2 overexpression is a p27Kip1-independent predictor of poor prognosis in patients with biliary tract cancers. Cancer Sci. 95, 969–976 (2004).
Traub, F., Mengel, M., Luck, H. J., Kreipe, H. H. & von Wasielewski, R. Prognostic impact of Skp2 and p27 in human breast cancer. Breast Cancer Res. Treat 99, 185–191 (2006).
Sonoda, H. et al. Significance of skp2 expression in primary breast cancer. Clin. Cancer Res. 12, 1215–1220 (2006).
Narayan, G. et al. Gene dosage alterations revealed by cDNA microarray analysis in cervical cancer: identification of candidate amplified and overexpressed genes. Genes Chromosomes Cancer 46, 373–384 (2007).
Gstaiger, M. et al. Skp2 is oncogenic and overexpressed in human cancers. Proc. Natl Acad. Sci. USA 98, 5043–5048 (2001).
Nishida, N., Nagasaka, T., Kashiwagi, K., Boland, C. R. & Goel, A. High copy amplification of the Aurora-A gene is associated with chromosomal instability phenotype in human colorectal cancers. Cancer Biol. Ther. 6, 525–533 (2007).
Kamata, Y. et al. High expression of skp2 correlates with poor prognosis in endometrial endometrioid adenocarcinoma. J. Cancer Res. Clin. Oncol. 131, 591–596 (2005).
Lahav-Baratz, S. et al. Decreased level of the cell cycle regulator p27 and increased level of its ubiquitin ligase Skp2 in endometrial carcinoma but not in normal secretory or in hyperstimulated endometrium. Mol. Hum. Reprod. 10, 567–572 (2004).
Ma, X. M., Liu, J. H., Guo, J. W., Liu, Y. & Zuo, L. F. Correlation of Skp2 expression in gastric carcinoma to expression of P27 and PTEN. Ai Zheng 25, 56–61 (2006).
Ma, X. M., Liu, Y., Guo, J. W., Liu, J. H. & Zuo, L. F. Relation of overexpression of S phase kinase-associated protein 2 with reduced expression of p27 and PTEN in human gastric carcinoma. World J. Gastroenterol. 11, 6716–6721 (2005).
Schiffer, D., Cavalla, P., Fiano, V., Ghimenti, C. & Piva, R. Inverse relationship between p27/Kip1 and the F-box protein Skp2 in human astrocytic gliomas by immunohistochemistry and western blot. Neurosci. Lett. 328, 125–128 (2002).
Saigusa, K. et al. Overexpressed Skp2 within 5p amplification detected by array-based comparative genomic hybridization is associated with poor prognosis of glioblastomas. Cancer Sci. 96, 676–683 (2005).
Penin, R. M. et al. Over-expression of p45SKP2 in Kaposi's sarcoma correlates with higher tumor stage and extracutaneous involvement but is not directly related to p27KIP1 down-regulation. Mod. Pathol. 15, 1227–1235 (2002).
Inui, N. et al. High expression of Cks1 in human non-small cell lung carcinomas. Biochem. Biophys. Res. Commun. 303, 978–984 (2003).
Yokoi, S. et al. Amplification and overexpression of SKP2 are associated with metastasis of non-small-cell lung cancers to lymph nodes. Am. J. Pathol. 165, 175–180 (2004).
Zhu, C. Q. et al. Skp2 gene copy number aberrations are common in non-small cell lung carcinoma, and its overexpression in tumors with ras mutation is a poor prognostic marker. Clin. Cancer Res. 10, 1984–1991 (2004).
Coe, B. P. et al. High-resolution chromosome arm 5p array CGH analysis of small cell lung carcinoma cell lines. Genes Chromosomes Cancer 42, 308–313 (2005).
Zhan, F. et al. CKS1B, overexpressed in aggressive disease, regulates multiple myeloma growth and survival through SKP2- and p27Kip1-dependent and -independent mechanisms. Blood 109, 4995–5001 (2007).
Shaughnessy, J. Amplification and overexpression of CKS1B at chromosome band 1q21 is associated with reduced levels of p27Kip1 and an aggressive clinical course in multiple myeloma. Hematology 10, S117–S126 (2005).
Woenckhaus, C. et al. Expression of Skp2 and p27KIP1 in naevi and malignant melanoma of the skin and its relation to clinical outcome. Histol. Histopathol 20, 501–508 (2005).
Li, Q., Murphy, M., Ross, J., Sheehan, C. & Carlson, J. A. Skp2 and p27kip1 expression in melanocytic nevi and melanoma: an inverse relationship. J. Cutan Pathol. 31, 633–642 (2004).
Katagiri, Y., Hozumi, Y. & Kondo, S. Knockdown of Skp2 by siRNA inhibits melanoma cell growth in vitro and in vivo. J. Dermatol. Sci. 42, 215–224 (2006).
Bhatt, K. V., Hu, R., Spofford, L. S. & Aplin, A. E. Mutant B-RAF signaling and cyclin D1 regulate Cks1/S-phase kinase-associated protein 2-mediated degradation of p27Kip1 in human melanoma cells. Oncogene 26, 1056–1066 (2007).
Fukuchi, M. et al. Inverse correlation between expression levels of p27 and the ubiquitin ligase subunit Skp2 in early esophageal squamous cell carcinoma. Anticancer Res. 24, 777–783 (2004).
Harada, K. et al. High expression of S-phase kinase-associated protein 2 (Skp2) is a strong prognostic marker in oral squamous cell carcinoma patients treated by UFT in combination with radiation. Anticancer Res. 25, 2471–2475 (2005).
Kudo, Y. et al. High expression of S-phase kinase-interacting protein 2, human F-box protein, correlates with poor prognosis in oral squamous cell carcinomas. Cancer Res. 61, 7044–7047 (2001).
Kitajima, S. et al. Role of Cks1 overexpression in oral squamous cell carcinomas: cooperation with Skp2 in promoting p27 degradation. Am. J. Pathol. 165, 2147–2155 (2004).
Shigemasa, K., Gu, L., O'Brien, T. J. & Ohama, K. Skp2 overexpression is a prognostic factor in patients with ovarian adenocarcinoma. Clin. Cancer Res. 9, 1756–1763 (2003).
Sui, L. et al. Clinical significance of Skp2 expression, alone and combined with Jab1 and p27 in epithelial ovarian tumors. Oncol. Rep. 15, 765–771 (2006).
Drobnjak, M. et al. Altered expression of p27 and Skp2 proteins in prostate cancer of African-American patients. Clin. Cancer Res. 9, 2613–2619 (2003).
Yang, G. et al. Elevated Skp2 protein expression in human prostate cancer: association with loss of the cyclin-dependent kinase inhibitor p27 and PTEN and with reduced recurrence-free survival. Clin. Cancer Res. 8, 3419–3426 (2002).
Amir, R. E., Haecker, H., Karin, M. & Ciechanover, A. Mechanism of processing of the NF-κB2 p100 precursor: identification of the specific polyubiquitin chain-anchoring lysine residue and analysis of the role of NEDD8-modification on the SCFβ-TrCP ubiquitin ligase. Oncogene 23, 2540–2547 (2004).
Fong, A. & Sun, S. C. Genetic evidence for the essential role of β-transducin repeat-containing protein in the inducible processing of NF-κB2/p100. J. Biol. Chem. 277, 22111–22114 (2002).
Lang, V. et al. βTrCP-mediated proteolysis of NF-κB1 p105 requires phosphorylation of p105 serines 927 and 932. Mol. Cell. Biol. 23, 402–413 (2003).
Orian, A. et al. SCFβ-TrCP ubiquitin ligase-mediated processing of NF-κB p105 requires phosphorylation of its C-terminus by IκB kinase. EMBO J. 19, 2580–2591 (2000).
Lassot, I. et al. ATF4 degradation relies on a phosphorylation-dependent interaction with the SCFβTrCP ubiquitin ligase. Mol. Cell. Biol. 21, 2192–2202 (2001).
Li, Y., Kumar, K. G., Tang, W., Spiegelman, V. S. & Fuchs, S. Y. Negative regulation of prolactin receptor stability and signaling mediated by SCFβ-TrC E3 ubiquitin ligase. Mol. Cell. Biol. 24, 4038–4048 (2004).
Besnard-Guerin, C. et al. HIV-1 Vpu sequesters β-transducin repeat-containing protein (βTrCP) in the cytoplasm and provokes the accumulation of β-catenin and other SCFβTrCP substrates. J. Biol. Chem. 279, 788–795 (2004).
Kumar, K. G., Krolewski, J. J. & Fuchs, S. Y. Phosphorylation and specific ubiquitin acceptor sites are required for ubiquitination and degradation of the IFNAR1 subunit of type I interferon receptor. J. Biol. Chem. 279, 46614–46620 (2004).
Mantovani, F. & Banks, L. Regulation of the discs large tumor suppressor by a phosphorylation-dependent interaction with the β-TrCP ubiquitin ligase receptor. J. Biol. Chem. 278, 42477–42486 (2003).
Reischl, S. et al. β-TrCP1-mediated degradation of PERIOD2 is essential for circadian dynamics. J. Biol. Rhythms 22, 375–386 (2007).
Shirogane, T., Jin, J., Ang, X. L. & Harper, J. W. SCFβ-TRCP controls clock-dependent transcription via casein kinase 1-dependent degradation of the mammalian period-1 (Per1) protein. J. Biol. Chem. 280, 26863–26872 (2005).
Eide, E. J. et al. Control of mammalian circadian rhythm by CKIε-regulated proteasome-mediated PER2 degradation. Mol. Cell. Biol. 25, 2795–2807 (2005).
Tian, Y. et al. TAZ promotes PC2 degradation through a SCFβ-Trcp E3 ligase complex. Mol. Cell. Biol. 27, 6383–6395 (2007).
Ding, Q. et al. Degradation of Mcl-1 by β-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol. Cell. Biol. 27, 4006–4017 (2007).
Tan, P. et al. Recruitment of a ROC1-CUL1 ubiquitin ligase by Skp1 and HOS to catalyze the ubiquitination of IκBα. Mol. Cell 3, 527–533 (1999).
Gallegos, J. R. et al. SCF TrCP1 activates and ubiquitylates TAp63γ. J. Biol. Chem. 283, 66–75 (2008).
van Kerkhof, P., Putters, J. & Strous, G. J. The ubiquitin ligase SCFβTrCP regulates the degradation of the growth hormone receptor. J. Biol. Chem. 282, 20475–20483 (2007).
Seki, A. et al. Plk1- and β-TrCP-dependent degradation of Bora controls mitotic progression. J. Cell Biol. 181, 65–78 (2008).
Soond, S. M. et al. ERK and the E3 ubiquitin ligase βTRCP targets STAT1 for degradation. J. Biol. Chem. (2008).
Acknowledgements
We thank S. Fuchs, Y. Ben-Neriah, K. Nakayama and J. Skaar for critically reading the manuscript. We apologize to colleagues whose work could not be mentioned owing to space limitations. D.F. is grateful to A. Nans. M.P. is grateful to T. M. Thor for continuous support. Work in the Pagano laboratory is supported by grants from the NIH (R37-CA76,584, R01-GM57,587, R21-CA125,173 and P30-CA01687) and the Multiple Myeloma Research Foundation senior award.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
National Cancer Institute
FURTHER INFORMATION
Glossary
- Ubiquitin
-
A small, 7.5-kDa protein that is ubiquitously expressed in all eukaryotes. Chains of ubiquitin moieties (connected by Lys48) target proteins for proteasomal degradation. Monoubiquitylation or polyubiquitylation through different lysine residues controls the function (not the proteolysis) of various proteins.
- Proteasome
-
A large multisubunit protein complex (approximately 2.5 MDa) that is found in all eukaryotes and archaea, the main function of which is to degrade excessive, unneeded or damaged proteins by proteolysis using a chemical reaction that breaks peptide bonds in an ATP-dependent manner.
- Ubiquitin-activating enzyme (E1)
-
An enzyme that activates ubiquitin in a process that requires ATP as an energy source.
- Ubiquitin-conjugating enzyme (E2)
-
An enzyme that accepts the transfer of ubiquitin from the ubiquitin-activating enzyme (E1) and transfers it to substrates.
- Ubiquitin ligase (E3)
-
An enzyme that functions as the substrate recognition component of the ubiquitylation machinery. E3 enzymes are capable of interacting with E2 enzymes and substrates to facilitate the transfer of ubiquitin to the selected substrate.
- RING-finger proteins
-
Proteins that interact with E2 ubiquitin enzymes to serve as an E3 enzyme. They are subdivided structurally into multi-subunit and single-subunit types, including those containing RING-like folds such as the U-box.
- HECT-domain proteins
-
Proteins that are characterized by the presence of a C-terminal HECT domain, which is a domain of approximately 350 amino acids that is catalytically involved in the attachment of ubiquitin to substrates.
- F-box domain
-
Originally identified in cyclin F as a stretch of approximately 40 amino acids linking F-box proteins to SKP1 to form the core of the SCF complex.
- Degron
-
Specific sequence of amino acids in a protein substrate typically conserved through evolution that directs the recognition of an E3 ubiquitin ligase.
- Paralogues
-
Homologous genes that have resulted from a gene duplication event within a single genome. This is in contrast to othologous genes, which are separated by a speciation event.
- C phase
-
The mammalian cell cycle is divided into four distinct phases called G1, S, G2 and mitosis. C phase is defined as the temporal interval between the G1–S transition and the end of mitosis when CDK activity is present.
- Organomegaly
-
The abnormal enlargement of organs.
- Mitotic catastrophe
-
A death resulting from failure of a cell to arrest before mitosis following DNA damage, resulting in severe aberrancies in chromosomal structure and segregation. It might share downstream events with apoptosis.
Rights and permissions
About this article
Cite this article
Frescas, D., Pagano, M. Deregulated proteolysis by the F-box proteins SKP2 and β-TrCP: tipping the scales of cancer. Nat Rev Cancer 8, 438–449 (2008). https://doi.org/10.1038/nrc2396
Issue Date:
DOI: https://doi.org/10.1038/nrc2396
This article is cited by
-
Skp2-mediated MLKL degradation confers cisplatin-resistant in non-small cell lung cancer cells
Communications Biology (2023)
-
Targeting the untargetable: RB1-deficient tumours are vulnerable to Skp2 ubiquitin ligase inhibition
British Journal of Cancer (2022)
-
Skp2 stabilizes Mcl-1 and confers radioresistance in colorectal cancer
Cell Death & Disease (2022)
-
Revealing β-TrCP activity dynamics in live cells with a genetically encoded biosensor
Nature Communications (2022)
-
Inability to phosphorylate Y88 of p27Kip1 enforces reduced p27 protein levels and accelerates leukemia progression
Leukemia (2022)