Key Points
-
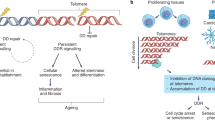
Telomeres are TTAGGG repetitive sequences that cap the ends of eukaryotic chromosomes.
-
A core of telomere binding proteins, termed the shelterin complex, serve to protect telomeric ends.
-
Critical telomere shortening or uncapping of telomere binding proteins results in telomere dysfunction.
-
Dysfunctional telomeres activate a DNA damage response. In the setting of a competent p53 pathway, this initiates senescence and apoptotic programmes to inhibit tumorigenesis.
-
In cells with mutant p53, dysfunctional telomeres promote genome instability and progression to cancer.
-
Cellular senescence is as potent as apoptosis in suppressing spontaneous tumorigenesis in mouse models of telomere dysfunction.
Abstract
Long-lived organisms such as humans have evolved several intrinsic tumour suppressor mechanisms to combat the slew of oncogenic somatic mutations that constantly arise in proliferating stem-cell compartments. One of these anticancer barriers is the telomere, a specialized nucleoprotein complex that caps the ends of eukaryotic chromosome. Impaired telomere function activates the canonical DNA damage response pathway that engages p53 to initiate apoptosis or replicative senescence. Here, we discuss how p53-dependent senescence induced by dysfunctional telomeres may be as potent as apoptosis in suppressing tumorigenesis in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961). A classic paper demonstrating that human cells have finite proliferative capacity in vitro (the 'Hayflick limit').
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995). This paper reports on the SA-β-gal assay as a way to mark senescent cells.
Bodnar, A. G. et al. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 (1998).
Vaziri, H. & Benchimol, S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr. Biol. 8, 279–282 (1998).
Maser, R. S. & DePinho, R. A. Connecting chromosomes, crisis, and cancer. Science 297, 565–569 (2002).
Masutomi, K. et al. Telomerase maintains telomere structure in normal human cells. Cell 114, 241–253 (2003).
Wright, W. E., Piatyszek, M. A., Rainey, W. E., Byrd, W. & Shay, J. W. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 18, 173–179 (1996).
Harley, C. B., Futcher, A. B. & Greider, C. W. Telomeres shorten during ageing of human fibroblasts. Nature 345, 458–460 (1990). This report links increasing telomere attrition with increased cell divisions and advancing age, suggesting that telomere shortening may be the underlying mechanism of the Hayflick limit.
Allsopp, R. C. et al. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl Acad. Sci. USA 89, 10114–10118 (1992).
Harley, C. B. et al. Telomerase, cell immortality, and cancer. Cold Spring Harb. Symp. Quant. Biol. 59, 307–315 (1994).
Blasco, M. A. Telomere length, stem cells and aging. Nature Chem. Biol. 3, 640–649 (2007).
Griffith, J. D. et al. Mammalian telomeres end in a large duplex loop. Cell 97, 503–514 (1999).
de Lange, T. T-loops and the origin of telomeres. Nature Rev. Mol. Cell Biol. 5, 323–329 (2004).
McClintock, B. the stability of broken ends of chromosomes in Zea Mays. Genetics 26, 234–282 (1941).
de Lange, T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 19, 2100–2110 (2005).
Liu, D., O'Connor, M. S., Qin, J. & Songyang, Z. Telosome, a mammalian telomere-associated complex formed by multiple telomeric proteins. J. Biol. Chem. 279, 51338–51342 (2004).
Verdun, R. E. & Karlseder, J. Replication and protection of telomeres. Nature 447, 924–931 (2007).
Corneo, B. et al. Rag mutations reveal robust alternative end joining. Nature 449, 483–486 (2007).
Yan, C. T. et al. IgH class switching and translocations use a robust non-classical end-joining pathway. Nature 449, 478–482 (2007).
Capper, R. et al. The nature of telomere fusion and a definition of the critical telomere length in human cells. Genes Dev. 21, 2495–2508 (2007).
DePinho, R. A. & Polyak, K. Cancer chromosomes in crisis. Nature Genet. 36, 932–934 (2004).
d'Adda di Fagagna, F. et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 (2003).
Takai, H., Smogorzewska, A. & de Lange, T. DNA damage foci at dysfunctional telomeres. Curr. Biol. 13, 1549–1556 (2003). References 22 and 23 report that dysfunctional telomeres activate a DDR, resulting in the accumulation of DDR proteins at telomeres. Cells with dysfunctional telomeres enter into senescence by activating a p53-dependent checkpoint response.
Wright, W. E. & Shay, J. W. The two-stage mechanism controlling cellular senescence and immortalization. Exp. Gerontol. 27, 383–389 (1992). This paper documents that DNA damage checkpoint proteins such as p53 and RB are required for cells with shortened telomeres to undergo cellular senescence. Elimination of these proteins enables these cells to immortalize.
Kamijo, T. et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91, 649–659 (1997).
Sage, J., Miller, A. L., Perez-Mancera, P. A., Wysocki, J. M. & Jacks, T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature 424, 223–228 (2003).
Guo, X. et al. Dysfunctional telomeres activate an ATM–ATR-dependent DNA damage response to suppress tumorigenesis. EMBO J. 26, 4709–4719 (2007).
Churikov, D. & Price, C. M. Pot1 and cell cycle progression cooperate in telomere length regulation. Nature Struct. Mol. Biol. 15, 79–84 (2008).
Gire, V., Roux, P., Wynford-Thomas, D., Brondello, J. M. & Dulic, V. DNA damage checkpoint kinase Chk2 triggers replicative senescence. EMBO J. 23, 2554–2563 (2004).
Denchi, E. L. & de Lange, T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature 448, 1068–1071 (2007).
Lee, H. W. et al. Essential role of mouse telomerase in highly proliferative organs. Nature 392, 569–574 (1998).
Rudolph, K. L. et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96, 701–712 (1999).
Rajaraman, S. et al. Telomere uncapping in progenitor cells with critical telomere shortening is coupled to S-phase progression in vivo. Proc. Natl Acad. Sci. USA 104, 17747–17752 (2007).
Hara, E., Tsurui, H., Shinozaki, A., Nakada, S. & Oda, K. Cooperative effect of antisense-Rb and antisense-p53 oligomers on the extension of life span in human diploid fibroblasts, TIG-1. Biochem. Biophys. Res. Commun. 179, 528–534 (1991).
Shay, J. W., Pereira-Smith, O. M. & Wright, W. E. A role for both RB and p53 in the regulation of human cellular senescence. Exp. Cell Res. 196, 33–39 (1991).
Counter, C. M. et al. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 11, 1921–1929 (1992).
Shay, J. W., Van Der Haegen, B. A., Ying, Y. & Wright, W. E. The frequency of immortalization of human fibroblasts and mammary epithelial cells transfected with SV40 large T-antigen. Exp. Cell Res. 209, 45–52 (1993).
Kim, N. W. et al. Specific association of human telomerase activity with immortal cells and cancer. Science 266, 2011–2015 (1994).
Bryan, T. M., Englezou, A., Dalla-Pozza, L., Dunham, M. A. & Reddel, R. R. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nature Med. 3, 1271–1274 (1997).
Shay, J. W. & Bacchetti, S. A survey of telomerase activity in human cancer. Eur. J. Cancer 33, 787–791 (1997).
Blasco, M. A., Funk, W., Villeponteau, B. & Greider, C. W. Functional characterization and developmental regulation of mouse telomerase RNA. Science 269, 1267–1270 (1995).
Blasco, M. A. et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91, 25–34 (1997).
Herrera, E., Martinez, A. C. & Blasco, M. A. Impaired germinal center reaction in mice with short telomeres. EMBO J. 19, 472–481 (2000).
Flores, I. et al. The longest telomeres: a general signature of adult stem cell compartments. Genes Dev. 22, 654–667 (2008).
Rossi, D. J. et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447, 725–729 (2007).
Chin, L. et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97, 527–538 (1999).
Artandi, S. E. et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406, 641–645 (2000).
Serrano, M. et al. Role of the INK4a locus in tumor suppression and cell mortality. Cell 85, 27–37 (1996).
Greenberg, R. A. et al. Short dysfunctional telomeres impair tumorigenesis in the INK4aΔ2/3 cancer-prone mouse. Cell 97, 515–525 (1999).
Gonzalez-Suarez, E., Samper, E., Flores, J. M. & Blasco, M. A. Telomerase-deficient mice with short telomeres are resistant to skin tumorigenesis. Nature Genet. 26, 114–117 (2000).
Rudolph, K. L., Millard, M., Bosenberg, M. W. & DePinho, R. A. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nature Genet. 28, 155–159 (2001).
Dove, W. F. et al. The intestinal epithelium and its neoplasms: genetic, cellular and tissue interactions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353, 915–923 (1998).
Farazi, P. A. et al. Differential impact of telomere dysfunction on initiation and progression of hepatocellular carcinoma. Cancer Res. 63, 5021–5027 (2003).
Collado, M. et al. Tumour biology: senescence in premalignant tumours. Nature 436, 642 (2005).
Braig, M. et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436, 660–665 (2005).
Collado, M., Blasco, M. A. & Serrano, M. Cellular senescence in cancer and aging. Cell 130, 223–233 (2007).
Feldser, D. M. & Greider, C. W. Short telomeres limit tumor progression in vivo by inducing senescence. Cancer Cell 11, 461–469 (2007).
Cosme-Blanco, W. et al. Telomere dysfunction suppresses spontaneous tumorigenesis in vivo by initiating p53-dependent cellular senescence. EMBO Rep. 8, 497–503 (2007). Using telomerase-knockout mouse models in a setting in which the apoptotic function of p53 is eliminated, references 57 and 58 document for the first time that activation of the cellular senescence programme could potently inhibit tumour initiation and progression in vivo.
Liu, G. et al. Chromosome stability, in the absence of apoptosis, is critical for suppression of tumorigenesis in Trp53 mutant mice. Nature Genet. 36, 63–68 (2004).
Ventura, A. et al. Restoration of p53 function leads to tumour regression in vivo. Nature 445, 661–665 (2007).
Choudhury, A. R. et al. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nature Genet. 39, 99–105 (2007).
Finkel, T., Serrano, M. & Blasco, M. A. The common biology of cancer and ageing. Nature 448, 767–774 (2007).
Harley, C. B. Telomerase and cancer therapeutics. Nature Rev. Cancer 8, 167–179 (2008).
Shay, J. W. & Keith, W. N. Targeting telomerase for cancer therapeutics. Br. J. Cancer 98, 677–683 (2008).
Damm, K. et al. A highly selective telomerase inhibitor limiting human cancer cell proliferation. EMBO J. 20, 6958–6968 (2001).
Dikmen, Z. G. et al. In vivo inhibition of lung cancer by GRN163L: a novel human telomerase inhibitor. Cancer Res. 65, 7866–7873 (2005).
Djojosubroto, M. W. et al. Telomerase antagonists GRN163 and GRN163L inhibit tumor growth and increase chemosensitivity of human hepatoma. Hepatology 42, 1127–1136 (2005).
Hochreiter, A. E. et al. Telomerase template antagonist GRN163L disrupts telomere maintenance, tumor growth, and metastasis of breast cancer. Clin. Cancer Res. 12, 3184–3192 (2006).
Jackson, S. R. et al. Antiadhesive effects of GRN163L — an oligonucleotide N3′→P5′ thio-phosphoramidate targeting telomerase. Cancer Res. 67, 1121–1129 (2007).
Ozawa, T. et al. Antitumor effects of specific telomerase inhibitor GRN163 in human glioblastoma xenografts. Neuro Oncol. 6, 218–226 (2004).
Salvati, E. et al. Telomere damage induced by the G-quadruplex ligand RHPS4 has an antitumor effect. J. Clin. Invest. 117, 3236–3247 (2007).
Campisi, J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 (2005).
Chin, K. et al. In situ analyses of genome instability in breast cancer. Nature Genet. 36, 984–988 (2004).
Meeker, A. K. et al. Telomere length abnormalities occur early in the initiation of epithelial carcinogenesis. Clin. Cancer Res. 10, 3317–3326 (2004).
Bartkova, J. et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434, 864–870 (2005).
Gorgoulis, V. G. et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434, 907–913 (2005).
Krtolica, A., Parrinello, S., Lockett, S., Desprez, P. Y. & Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl Acad. Sci. USA 98, 12072–12077 (2001).
Baumann, P. & Cech, T. R. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292, 1171–1175 (2001).
He, H. et al. POT1b protects telomeres from end-to-end chromosomal fusions and aberrant homologous recombination. EMBO J. 25, 5180–5190 (2006).
Loayza, D. & De Lange, T. POT1 as a terminal transducer of TRF1 telomere length control. Nature 423, 1013–1018 (2003).
Wu, L. et al. Pot1 deficiency initiates DNA damage checkpoint activation and aberrant homologous recombination at telomeres. Cell 126, 49–62 (2006).
Wang, F. et al. The POT1–TPP1 telomere complex is a telomerase processivity factor. Nature 445, 506–510 (2007).
Xin, H. et al. TPP1 is a homologue of ciliate TEBP-β and interacts with POT1 to recruit telomerase. Nature 445, 559–562 (2007).
Hockemeyer, D., Daniels, J. P., Takai, H. & de Lange, T. Recent expansion of the telomeric complex in rodents: two distinct POT1 proteins protect mouse telomeres. Cell 126, 63–77 (2006).
Hockemeyer, D. et al. Telomere protection by mammalian Pot1 requires interaction with Tpp1. Nature Struct. Mol. Biol. 14, 754–761 (2007).
Celli, G. B. & de Lange, T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nature Cell Biol. 7, 712–718 (2005).
Chiang, Y. J., Kim, S. H., Tessarollo, L., Campisi, J. & Hodes, R. J. Telomere-associated protein TIN2 is essential for early embryonic development through a telomerase-independent pathway. Mol. Cell Biol. 24, 6631–6634 (2004).
Munoz, P., Blanco, R. & Blasco, M. A. Role of the TRF2 telomeric protein in cancer and ageing. Cell Cycle 5, 718–721 (2006).
Kim, W. Y. & Sharpless, N. E. The regulation of INK4/ARF in cancer and aging. Cell 127, 265–275 (2006).
Jacobs, J. J. & de Lange, T. Significant role for p16INK4A in p53-independent telomere-directed senescence. Curr. Biol. 14, 2302–2308 (2004).
Herbig, U., Jobling, W. A., Chen, B. P., Chen, D. J. & Sedivy, J. M. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21CIP1, but not p16INK4a. Mol. Cell 14, 501–513 (2004).
Smogorzewska, A. & de Lange, T. Different telomere damage signaling pathways in human and mouse cells. EMBO J. 21, 4338–4348 (2002).
Khoo, C. M., Carrasco, D. R., Bosenberg, M. W., Paik, J. H. & Depinho, R. A. Ink4a/Arf tumor suppressor does not modulate the degenerative conditions or tumor spectrum of the telomerase-deficient mouse. Proc. Natl Acad. Sci. USA 104, 3931–3936 (2007).
Siegl-Cachedenier, I., Munoz, P., Flores, J. M., Klatt, P. & Blasco, M. A. Deficient mismatch repair improves organismal fitness and survival of mice with dysfunctional telomeres. Genes Dev. 21, 2234–2247 (2007).
Qi, L. et al. Short telomeres and ataxia-telangiectasia mutated deficiency cooperatively increase telomere dysfunction and suppress tumorigenesis. Cancer Res. 63, 8188–8196 (2003).
Wong, K. K. et al. Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature 421, 643–648 (2003).
Maser, R. S. et al. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature 447, 966–971 (2007).
Blanco, R., Munoz, P., Flores, J. M., Klatt, P. & Blasco, M. A. Telomerase abrogation dramatically accelerates TRF2-induced epithelial carcinogenesis. Genes Dev. 21, 206–220 (2007).
Acknowledgements
S.C acknowledges generous financial support from the NIA (RO1 AG028888), the NCI (RO1 CA129037), the Welch Foundation, the Elsa U. Pardee Foundation, the Sydney Kimmel Foundation for Cancer Research, the Abraham and Phyllis Katz Foundation, and the Michael Kadoorie Cancer Genetic Research Program. Y.D. is supported by a NCI Howard Temin Award (1K01CA124461) and S.S.C is supported by a NIH Predoctoral Training Grant.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
National Cancer Institute
National Cancer Institute Drug Dictionary
FURTHER INFORMATION
Glossary
- Breakage–fusion–bridge cycle
-
Chromosomal ends with critically shortened telomeres are highly recombinogenic, and undergo repeated cycles of end-to-end fusions, followed by random breakage, and then subsequent fusions to generate loss of heterozygosity or amplification of certain chromosomal loci.
- Dicentric chromosome
-
A chromosome that has two centromeres, formed by breakage and reunion of two chromosomes.
Rights and permissions
About this article
Cite this article
Deng, Y., Chan, S. & Chang, S. Telomere dysfunction and tumour suppression: the senescence connection. Nat Rev Cancer 8, 450–458 (2008). https://doi.org/10.1038/nrc2393
Issue Date:
DOI: https://doi.org/10.1038/nrc2393
This article is cited by
-
Associations between telomere attrition, genetic variants in telomere maintenance genes, and non-small cell lung cancer risk in the Jammu and Kashmir population of North India
BMC Cancer (2023)
-
Three-dimensional nuclear telomere architecture and differential expression of aurora kinase genes in chronic myeloid leukemia to measure cell transformation
BMC Cancer (2022)
-
Impact of superovulation and in vitro fertilization on LINE-1 copy number and telomere length in C57BL/6 J mice blastocysts
Molecular Biology Reports (2022)
-
Human skin aging is associated with increased expression of the histone variant H2A.J in the epidermis
npj Aging and Mechanisms of Disease (2021)
-
Exploring the multiple roles of guardian of the genome: P53
Egyptian Journal of Medical Human Genetics (2020)