Key Points
-
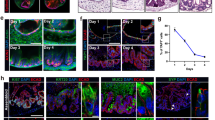
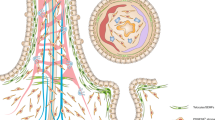
Intestinal crypts house the tissue-specific, multipotential stem cells located in the niche at the base of the crypt, capable of regenerating all intestinal cell types.
-
Intestinal crypts are clonal populations.
-
A stem cell that has acquired a mutation can expand to occupy the whole stem-cell niche, through genetic drift or by acquiring a selective advantage (niche succession); eventually their progeny can fill the whole crypt (monoclonal conversion).
-
Mutated intestinal crypts expand by crypt fission, leading to spread of the mutation within the epithelium (field cancerization).
-
Further genetic changes result in the appearance of the earliest recognizable lesion in the colon (the monocryptal adenoma); expansion by crypt fission results in the development of the premalignant lesion in the colon (the adenoma).
-
Adenomas appear to be polyclonal; short-range interactions between adjacent initiated clones might be responsible, with clonal interaction required for adenoma formation.
-
Although it is clear that many tumours arise from stem cells, there is early evidence that committed progenitor cells linger long enough in the crypt to undergo mutation and selection events that lead to neoplasia.
-
These concepts can be applied to other tissues and organs as a basis for the development of the earliest neoplastic lesions.
Abstract
An appreciation of colonic crypt organization has become essential to any understanding of tumorigenesis in the colon. Intestinal crypts house tissue-specific, multipotential stem cells, which are located in the niche at the base of the intestinal crypt and are capable of regenerating all intestinal cell types. Recent advances in our understanding of crypt biology, including how mutations in stem cells become fixed and expand within the epithelium, has led to new theories on the origins of colonic adenomas and cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Moolgavkar, S. H. & Luebeck, E. G. Multistage carcinogenesis and the incidence of human cancer. Genes Chromosomes Cancer 38, 302–306 (2003).
Dakubo, G. D., Jakupciak, J. P., Birch-Machin, M. A. & Parr, R. L. Clinical implications and utility of field cancerization. Cancer Cell Int. 7, 2 (2007).
Vatve, M., Ortonne, J. P., Birch-Machin, M. A. & Gupta, G. Management of field change in actinic keratosis. Br. J. Dermatol. 157 (Suppl 2), 21–24 (2007).
Loeffler, M. et al. Dose–response relationship of complementary radiotherapy following four cycles of combination chemotherapy in intermediate-stage Hodgkin's disease. J. Clin. Oncol. 15, 2275–2287 (1997).
Hornick, J. L. & Odze, R. D. Neoplastic precursor lesions in Barrett's esophagus. Gastroenterol. Clin. North Am. 36, 775–796 (2007).
Boland, C. R. & Goel, A. Somatic evolution of cancer cells. Semin. Cancer Biol. 15, 436–450 (2005).
Durie, B. G. & Salmon, S. E. Cell kinetic analysis of human tumor stem cells. Prog. Clin. Biol. Res. 48, 153–163 (1980).
Fevr, T., Robine, S., Louvard, D. & Huelsken, J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol. Cell Biol. 27, 7551–7559 (2007).
Kosinski, C. et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc. Natl Acad. Sci. USA 104, 15418–15423 (2007).
Crosnier, C., Stamataki, D. & Lewis, J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nature Rev. Genet. 7, 349–359 (2006).
van Es, J. H. et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435, 959–963 (2005).
Batlle, E. et al. β-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111, 251–263 (2002).
Cheng, H. & Leblond, C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am. J. Anat. 141, 537–561 (1974).
Ponder, B. A. et al. Derivation of mouse intestinal crypts from single progenitor cells. Nature 313, 689–691 (1985). Provided evidence that, in the mouse, intestinal crypts are clonal populations derived from a multipotential stem cell
Thompson, M. et al. Gastric endocrine cells share a clonal origin with other gut cell lineages. Development 110, 477–481 (1990).
Bjerknes, M. & Cheng, H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology 116, 7–14 (1999).
Bjerknes, M. & Cheng, H. Multipotential stem cells in adult mouse gastric epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G767–G777 (2002).
Dekaney, C. M., Rodriguez, J. M., Graul, M. C. & Henning, S. J. Isolation and characterization of a putative intestinal stem cell fraction from mouse jejunum. Gastroenterology 129, 1567–1580 (2005).
May, R. et al. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells 26, 630–637 (2008).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007). A key paper that identified Lgr5 as a marker for intestinal cells capable of clonal conversion, that is, stem cells, in a mouse model.
Qiao, X. T. et al. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology 133, 1989–1998 (2007).
Novelli, M. R. et al. Polyclonal origin of colonic adenomas in an XO/XY patient with FAP. Science 272, 1187–1190 (1996). This paper demonstrated that human colonic crypts are clonal populations; adenomas were found to be predominantly polyclonal.
Novelli, M. et al. X-inactivation patch size in human female tissue confounds the assessment of tumor clonality. Proc. Natl Acad. Sci. USA 100, 3311–3314 (2003).
Campbell, F. et al. Post-irradiation somatic mutation and clonal stabilisation time in the human colon. Gut 39, 569–573 (1996).
Taylor, R. W. et al. Mitochondrial DNA mutations in human colonic crypt stem cells. J. Clin. Invest. 112, 1351–1360 (2003).
McDonald, S. A. et al. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology 134, 500–510 (2008).
Meineke, F. A., Potten, C. S. & Loeffler, M. Cell migration and organization in the intestinal crypt using a lattice-free model. Cell Prolif. 34, 253–266 (2001).
Ohlstein, B., Kai, T., Decotto, E. & Spradling, A. The stem cell niche: theme and variations. Curr. Opin. Cell Biol. 16, 693–699 (2004).
Li, L. & Xie, T. Stem cell niche: structure and function. Annu. Rev. Cell Dev. Biol. 21, 605–631 (2005).
Yamashita, Y. M., Fuller, M. T. & Jones, D. L. Signaling in stem cell niches: lessons from the Drosophila germline. J. Cell Sci. 118, 665–672 (2005).
Xie, T. & Spradling, A. C. A niche maintaining germ line stem cells in the Drosophila ovary. Science 290, 328–330 (2000).
Kim, K. M. & Shibata, D. Methylation reveals a niche: stem cell succession in human colon crypts. Oncogene 21, 5441–5449 (2002). This paper provides valuable insights into the dynamics of the stem cell niche in humans.
Nicolas, P., Kim, K. M., Shibata, D. & Tavare, S. The stem cell population of the human colon crypt: analysis via methylation patterns. PLoS Comput. Biol. 3, e28 (2007).
Ahuja, N., Li, Q., Mohan, A. L., Baylin, S. B. & Issa, J. P. Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res. 58, 5489–5494 (1998).
Issa, J. P. CpG-island methylation in aging and cancer. Curr. Top. Microbiol. Immunol. 249, 101–118 (2000).
Maley, C. C. et al. Selectively advantageous mutations and hitchhikers in neoplasms: p16 lesions are selected in Barrett's esophagus. Cancer Res. 64, 3414–3427 (2004).
Kodama, M. et al. Expression of mutant type-p53 products in H. pylori-associated chronic gastritis. World J. Gastroenterol. 13, 1541–1546 (2007).
Liu, Q. et al. CDX2 expression is progressively decreased in human gastric intestinal metaplasia, dysplasia and cancer. Mod. Pathol. 20, 1286–1297 (2007).
Bernstein, C. N. Neoplasia in inflammatory bowel disease: surveillance and management strategies. Curr. Gastroenterol. Rep. 8, 513–518 (2006).
Itzkowitz, S. H. & Yio, X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G7–G17 (2004).
Brentnall, T. A. et al. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology 107, 369–378 (1994).
Karpowicz, P. et al. Support for the immortal strand hypothesis: neural stem cells partition DNA asymmetrically in vitro. J. Cell Biol. 170, 721–732 (2005).
Burmer, G. C. et al. Neoplastic progression in ulcerative colitis: histology, DNA content, and loss of a p53 allele. Gastroenterology 103, 1602–1610 (1992).
Levine, D. S. et al. Distribution of aneuploid cell populations in ulcerative colitis with dysplasia or cancer. Gastroenterology 101, 1198–1210 (1991).
Greaves, L. C. et al. Mitochondrial DNA mutations are established in human colonic stem cells, and mutated clones expand by crypt fission. Proc. Natl Acad. Sci. USA 103, 714–719 (2006). Compelling evidence that crypt fission is the mechanism by which human colonic crypts divide.
Ushijima, T. Epigenetic field for cancerization. J. Biochem. Mol. Biol. 40, 142–150 (2007).
Maley, C. C. Multistage carcinogenesis in Barrett's esophagus. Cancer Lett. 245, 22–32 (2007).
Merlo, L. M., Pepper, J. W., Reid, B. J. & Maley, C. C. Cancer as an evolutionary and ecological process. Nature Rev. Cancer 6, 924–935 (2006).
Coad, R. A. et al. On the histogenesis of Barrett's oesophagus and its associated squamous islands: a three-dimensional study of their morphological relationship with native oesophageal gland ducts. J. Pathol. 206, 388–394 (2005).
Wasan, H. S. et al. APC in the regulation of intestinal crypt fission. J. Pathol. 185, 246–255 (1998).
Vogelstein, B. et al. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 319, 525–532 (1988).
Boman, B. M. et al. Colonic crypt changes during adenoma development in familial adenomatous polyposis: immunohistochemical evidence for expansion of the crypt base cell population. Am. J. Pathol. 165, 1489–1498 (2004).
Loeffler, M., Bratke, T., Paulus, U., Li, Y. Q. & Potten, C. S. Clonality and life cycles of intestinal crypts explained by a state dependent stochastic model of epithelial stem cell organization. J. Theor. Biol. 186, 41–54 (1997).
Lamlum, H. et al. APC mutations are sufficient for the growth of early colorectal adenomas. Proc. Natl Acad. Sci. USA 97, 2225–2228 (2000).
Luebeck, E. G. & Moolgavkar, S. H. Multistage carcinogenesis and the incidence of colorectal cancer. Proc. Natl Acad. Sci. USA 99, 15095–15100 (2002).
Nakamura, S. & Kino, I. Morphogenesis of minute adenomas in familial polyposis coli. J. Natl Cancer Inst. 73, 41–49 (1984).
Woda, B. A., Forde, K. & Lane, N. A unicryptal colonic adenoma, the smallest colonic neoplasm yet observed in a non-polyposis individual. Am. J. Clin. Pathol. 68, 631–632 (1977).
Shih, I. M. et al. Top-down morphogenesis of colorectal tumors. Proc. Natl Acad. Sci. USA 98, 2640–2645 (2001).
Preston, S. L. et al. Bottom-up histogenesis of colorectal adenomas: origin in the monocryptal adenoma and initial expansion by crypt fission. Cancer Res. 63, 3819–3825 (2003). Conclusive proof that crypt fission is the predominant mode of spread of adenomatous crypts.
Wong, W. M. et al. Histogenesis of human colorectal adenomas and hyperplastic polyps: the role of cell proliferation and crypt fission. Gut 50, 212–217 (2002).
van den Brink, G. R. & Offerhaus, G. J. The morphogenetic code and colon cancer development. Cancer Cell 11, 109–117 (2007).
Roncucci, L., Medline, A. & Bruce, W. R. Classification of aberrant crypt foci and microadenomas in human colon. Cancer Epidemiol. Biomarkers Prev. 1, 57–60 (1991).
Jen, J. et al. Molecular determinants of dysplasia in colorectal lesions. Cancer Res. 54, 5523–5526 (1994).
Takayama, T. et al. Analysis of K-ras, APC, and β-catenin in aberrant crypt foci in sporadic adenoma, cancer, and familial adenomatous polyposis. Gastroenterology 121, 599–611 (2001).
Jass, J. R., Whitehall, V. L., Young, J. & Leggett, B. A. Emerging concepts in colorectal neoplasia. Gastroenterology 123, 862–876 (2002).
Tsao, J. L. et al. Tracing cell fates in human colorectal tumors from somatic microsatellite mutations: evidence of adenomas with stem cell architecture. Am. J. Pathol. 153, 1189–1200 (1998).
Fialkow, P. J. Clonal origin of human tumors. Biochim. Biophys. Acta 458, 283–321 (1976).
Hsu, S. H., Luk, G. D., Krush, A. J., Hamilton, S. R. & Hoover, H. H. Jr. Multiclonal origin of polyps in Gardner syndrome. Science 221, 951–953 (1983).
Newton, M. A. On estimating the polyclonal fraction in lineage-marker studies of tumor origin. Biostatistics 7, 503–514 (2006).
Merritt, A. J., Gould, K. A. & Dove, W. F. Polyclonal structure of intestinal adenomas in ApcMin/+ mice with concomitant loss of Apc+ from all tumor lineages. Proc. Natl Acad. Sci. USA 94, 13927–13931 (1997).
Axelrod, R., Axelrod, D. E. & Pienta, K. J. Evolution of cooperation among tumor cells. Proc. Natl Acad. Sci. USA 103, 13474–13479 (2006).
Thliveris, A. T. et al. Polyclonality of familial murine adenomas: analyses of mouse chimeras with low tumor multiplicity suggest short-range interactions. Proc. Natl Acad. Sci. USA 102, 6960–6965 (2005).
Ishiguro, K., Yoshida, T., Yagishita, H., Numata, Y. & Okayasu, T. Epithelial and stromal genetic instability contributes to genesis of colorectal adenomas. Gut 55, 695–702 (2006).
Bian, Y. et al. Somatic acquisition of TGFBR1*6A by epithelial and stromal cells during head and neck and colon cancer development. Hum. Mol. Genet. 16, 3128–3135 (2007).
O'Brien, C. A., Pollett, A., Gallinger, S. & Dick, J. E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445, 106–110 (2007).
Ricci-Vitiani, L. et al. Identification and expansion of human colon-cancer-initiating cells. Nature 445, 111–115 (2007).
Lamprecht, S. A. & Lipkin, M. Migrating colonic crypt epithelial cells: primary targets for transformation. Carcinogenesis 23, 1777–1780 (2002).
Makino, T. et al. Primary signet-ring cell carcinoma of the colon and rectum: report of eight cases and review of 154 Japanese cases. Hepatogastroenterology 53, 845–849 (2006).
Vogelsang, H. & Siewert, J. R. Endocrine tumours of the hindgut. Best Pract. Res. Clin. Gastroenterol. 19, 739–751 (2005).
Rubio, C. A., Kanter, L., Bjork, J., Poppen, B. & Bry, L. Paneth cell-rich flat adenoma of the rectum: report of a case. Jpn. J. Cancer Res. 87, 109–112 (1996).
Pierce, G. B. & Speers, W. C. Tumors as caricatures of the process of tissue renewal: prospects for therapy by directing differentiation. Cancer Res. 48, 1996–2004 (1988).
Houghton, J. & Wang, T. C. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology 128, 1567–1578 (2005).
Park, H. S., Goodlad, R. A. & Wright, N. A. Crypt fission in the small intestine and colon. A mechanism for the emergence of G6PD locus-mutated crypts after treatment with mutagens. Am. J. Pathol. 147, 1416–1427 (1995).
Williams, E. D., Lowes, A. P., Williams, D. & Williams, G. T. A stem cell niche theory of intestinal crypt maintenance based on a study of somatic mutation in colonic mucosa. Am. J. Pathol. 141, 773–776 (1992). A seminal paper that first proposed the theory that intestinal crypts contain multiple stem cells housed within a stem cell niche.
Yatabe, Y., Tavare, S. & Shibata, D. Investigating stem cells in human colon by using methylation patterns. Proc. Natl Acad. Sci. USA 98, 10839–10844 (2001).
Cheng, H., Bjerknes, M., Amar, J. & Gardiner, G. Crypt production in normal and diseased human colonic epithelium. Anat. Rec. 216, 44–48 (1986).
Totafurno, J., Bjerknes, M. & Cheng, H. The crypt cycle. Crypt and villus production in the adult intestinal epithelium. Biophys. J. 52, 279–294 (1987).
Kim, K. M. & Shibata, D. Tracing ancestry with methylation patterns: most crypts appear distantly related in normal adult human colon. BMC Gastroenterol. 4, 8 (2004).
Acknowledgements
We are grateful to S. McDonald, S. Leedham, P. Tadrous and D. Turnbull for helpful discussions and for providing figures. This work was supported by Cancer Research UK.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
National Cancer Institute
Glossary
- Actinic keratosis
-
A dysplastic condition of the skin secondary to chronic sunlight exposure, which predisposes to squamous cell carcinoma.
- Squamous metaplasia
-
The abnormal change from one defined epithelium to a squamous epithelium.
- Pericryptal myofibroblasts
-
Mesenchymal cells that surround and support the intestinal crypt, derived from the myofibroblast lineage.
- Paneth cells
-
Differentiated intestinal epithelial cells, mainly located in the small intestine and occasionally found in the ascending colon, that contain eosinophilic granules and secrete antibacterial proteins.
- Hypomorphic variant
-
A variant with a reduced function compared with the wild type.
Rights and permissions
About this article
Cite this article
Humphries, A., Wright, N. Colonic crypt organization and tumorigenesis. Nat Rev Cancer 8, 415–424 (2008). https://doi.org/10.1038/nrc2392
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2392