Key Points
-
In order to assure proper coverage of the clinical target volume (CTV) by radiation, a margin needs to be added to compensate for daily positioning errors and internal motion of organs, resulting in the planning target volume (PTV). The PTV therefore includes normal tissues near the tumour, to which radiation is intentionally delivered.
-
The dose of radiotherapy that is necessary to control a tumour is often not delivered because of a high probability of complications in nearby normal tissues. This problem can be tackled by the generation of conformal dose distributions that tightly match the volume of the PTV and/or by decreasing the amount of normal tissue in the PTV.
-
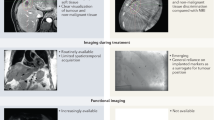
Image-guided radiotherapy (IGRT) is defined as frequent imaging in the treatment room that allows treatment decisions to be made on the basis of these images. IGRT aims at decreasing CTV-to-PTV margins from centimetres to millimetres.
-
The synergy between conformal radiotherapy (CRT) and IGRT has drastically improved the quality of radiotherapy and has broadened its possibilities and indications. Clinical implementations of CRT–IGRT have enabled dose escalation, conformal sparing and non-uniform dose distributions, and initiated a revision of fractionation schedules.
-
Research to improve image quality in radiotherapy is not new, but developments of software to quantify target localization errors, on the basis of in-room imaging and hardware allowing automated set-up, have stimulated mainstream clinical application of IGRT.
-
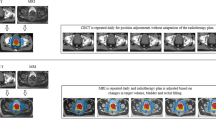
IGRT makes use of many different imaging techniques, using modalities ranging from planar imaging to fluoroscopy to cone-beam CT, and following procedures as simple as using a single set-up image or as complex as intra-fraction tumour tracking.
-
IGRT can be applied for managing of inter-fraction as well as intra-fraction geometric set-up uncertainties and for adapting treatments to tumour responses.
Abstract
The limited ability to control for the location of a tumour compromises the accuracy with which radiation can be delivered to tumour-bearing tissue. The resultant requirement for larger treatment volumes to accommodate target uncertainty restricts the radiation dose because more surrounding normal tissue is exposed. With image-guided radiotherapy (IGRT) these volumes can be optimized and tumoricidal doses can be delivered, achieving maximal tumour control with minimal complications. Moreover, with the ability of high-precision dose delivery and real-time knowledge of the target volume location, IGRT has initiated the exploration of new indications for radiotherapy, some of which were previously considered infeasible.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brenner, D. J., Hlatky, L. R., Hahnfeldt, P. J., Huang, Y. & Sachs, R. K. The linear-quadratic model and most other common radiobiological models result in similar predictions of time-dose relationships. Radiat. Res. 150, 83–91 (1998).
International Commission on Radiation Units and Measurements. Prescribing, Recording and Reporting Photon Beam Therapy, Report 50 (ICRU, Bethesda, 1993).
International Commission on Radiation Units and Measurements. Prescribing, Recording and Reporting Photon Beam Therapy, Report 62 (ICRU, Bethesda, 1999). References 2 and 3 describe the rationale behind the concept of treatment margins in radiotherapy and provide a clear definition of the different volumes.
Wang, D. et al. Initial experience of FDG-PET/CT guided IMRT of head-and-neck carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 65, 143–151 (2006).
Balter, J. M. & Kessler, M. L. Imaging and alignment for image-guided radiation therapy. J. Clin. Oncol. 25, 931–937 (2007). This review explores the issues surrounding the use of images and image registration for treatment planning and treatment verification in radiotherapy.
Gregoire, V. Is there any future in radiotherapy planning without the use of PET: unraveling the myth. Radiother. Oncol. 73, 261–263 (2004).
Rothschild, S. et al. PET/CT staging followed by Intensity-Modulated Radiotherapy (IMRT) improves treatment outcome of locally advanced pharyngeal carcinoma: a matched-pair comparison. Radiat. Oncol. 2, 22 (2007).
Bernier, J., Hall, E. J. & Giaccia, A. Radiation oncology: a century of achievements. Nature Rev. Cancer 4, 737–747 (2004). In this excellent review Bernier and colleagues highlight the progress of radiation therapy in the twentieth century with emphasis on the refinements of irradiation techniques and radiobiology. This Review and Bernier's review are complementary.
Bel, A., Van Herk, M. & Lebesque, J. V. Target margins for random geometrical treatment uncertainties in conformal radiotherapy. Med. Phys. 23, 1537–1545 (1996).
Bel, A. et al. High-precision prostate cancer irradiation by clinical application of an offline patient setup verification procedure, using portal imaging. Int. J. Radiat. Oncol. Biol. Phys. 35, 321–332 (1996).
Van Herk, M. et al. Quantification of organ motion during conformal radiotherapy of the prostate by three dimensional image registration. Int. J. Radiat. Oncol. Biol. Phys. 33, 1311–1320 (1995).
Yan, D., Wong, J. W., Gustafson, G. & Martinez, A. A new model for 'accept or reject' strategies in off-line and on-line megavoltage treatment evaluation. Int. J. Radiat. Oncol. Biol. Phys. 31, 943–952 (1995).
Yan, D. et al. Adaptive modification of treatment planning to minimize the deleterious effects of treatment setup errors. Int. J. Radiat. Oncol. Biol. Phys. 38, 197–206 (1997).
Alasti, H., Petric, M. P., Catton, C. N. & Warde, P. R. Portal imaging for evaluation of daily on-line setup errors and off-line organ motion during conformal irradiation of carcinoma of the prostate. Int. J. Radiat. Oncol. Biol. Phys. 49, 869–884 (2001).
De Neve, W. et al. Interactive use of on-line portal imaging in pelvic radiation. Int. J. Radiat. Oncol. Biol. Phys. 25, 517–524 (1993).
Gildersleve, J. et al. A randomised trial of patient repositioning during radiotherapy using a megavoltage imaging system. Radiother. Oncol. 31, 161–168 (1994).
Schaake-Koning, C. et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N. Engl. J. Med. 326, 524–530 (1992).
Herskovic, A. et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N. Engl. J. Med. 326, 1593–1598 (1992).
Krook, J. E. et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N. Engl. J. Med. 324, 709–715 (1991).
De Ridder, M. et al. Lipid a radiosensitizes hypoxic EMT-6 tumor cells: role of the NF-kappaB signaling pathway. Int. J. Radiat. Oncol. Biol. Phys. 57, 779–786 (2003).
Brown, J. M. & Wilson, W. R. Exploiting tumour hypoxia in cancer treatment. Nature Rev. Cancer 4, 437–447 (2004).
De Ridder, M. et al. Macrophages enhance the radiosensitizing activity of lipid A: a novel role for immune cells in tumor cell radioresponse. Int. J. Radiat. Oncol. Biol. Phys. 60, 598–606 (2004).
Bonner, J. A. et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 354, 567–578 (2006).
De Ridder, M. et al. The radiosensitizing effect of immunoadjuvant OM-174 requires cooperation between immune and tumor cells through interferon-gamma and inducible nitric oxide synthase. Int. J. Radiat. Oncol. Biol. Phys. 66, 1473–1480 (2006).
Nyati, M. K., Morgan, M. A., Feng, F. Y. & Lawrence, T. S. Integration of EGFR inhibitors with radiochemotherapy. Nature Rev. Cancer 6, 876–885 (2006).
Czito, B. G. et al. Bevacizumab, oxaliplatin, and capecitabine with radiation therapy in rectal cancer: Phase I trial results. Int. J. Radiat. Oncol. Biol. Phys. 68, 472–478 (2007).
Intensity Modulated Radiation Therapy Collaborative Working Group. Intensity-modulated radiotherapy: current status and issues of interest. Int. J. Radiat. Oncol. Biol. Phys. 51, 880–914 (2001). A critical review describing the state-of-the-art in IMRT in 2001.
Peeters, S. T. et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J. Clin. Oncol. 24, 1990–1996 (2006).
Pollack, A. et al. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int. J. Radiat. Oncol. Biol. Phys. 64, 518–526 (2006).
Kupelian, P. A. et al. Radical prostatectomy, external beam radiotherapy <72 Gy, external beam radiotherapy > or =72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1-T2 prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 58, 25–33 (2004). This retrospective analysis of nearly 3,000 patients illustrates that dose escalation can improve the outcome of radiotherapy, which might therefore become an alternative to surgery.
Pow, E. H. et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: Initial report on a randomized controlled clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 66, 981–991 (2006).
De Ridder, M. et al. Phase II study of preoperative helical tomotherapy for rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. (in the press).
Emami, B. et al. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 21, 109–122 (1991).
Tome, W. A. & Fowler, J. F. Selective boosting of tumor subvolumes. Int. J. Radiat. Oncol. Biol. Phys. 48, 593–599 (2000).
Deasy, J. O. Partial tumor boosts: even more attractive than theory predicts? Int. J. Radiat. Oncol. Biol. Phys. 51, 279–280 (2001).
Gambhir, S. S. Molecular imaging of cancer with positron emission tomography. Nature Rev. Cancer 2, 683–693 (2002).
Payne, G. S. & Leach, M. O. Applications of magnetic resonance spectroscopy in radiotherapy treatment planning. Br. J. Radiol. 79, S16–S26 (2006).
Thorwarth, D., Eschmann, S. M., Paulsen, F. & Alber, M. Hypoxia dose painting by numbers: a planning study. Int. J. Radiat. Oncol. Biol. Phys. 68, 291–300 (2007).
Ling, C. C. et al. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int. J. Radiat. Oncol. Biol. Phys. 47, 551–560 (2000). This critical review summarizes the advances in imaging that have potential applications in radiation oncology, and explores the concept of integrating physical and biological conformality in CRT.
Bentzen, S. M. Theragnostic imaging for radiation oncology: dose-painting by numbers. Lancet Oncol. 6, 112–117 (2005).
Fletcher, G. H. Hypofractionation: lessons from complications. Radiother. Oncol. 20, 10–15 (1991).
Harrison, D., Crennan, E., Cruickshank, D., Hughes, P. & Ball, D. Hypofractionation reduces the therapeutic ratio in early glottic carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 15, 365–372 (1988).
Kim, J. J. & Tannock, I. F. Repopulation of cancer cells during therapy: an important cause of treatment failure. Nature Rev. Cancer 5, 516–525 (2005).
Herfarth, K. K. et al. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J. Clin. Oncol 19, 164–170 (2001).
Nagata, Y. et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int. J. Radiat. Oncol. Biol. Phys. 63, 1427–1431 (2005).
Xia, T. et al. Promising clinical outcome of stereotactic body radiation therapy for patients with inoperable Stage I/II non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 66, 117–125 (2006).
Haus, A. G., Pinsky, S. M. & Marks, J. E. A technique for imaging patient treatment area during a therapeutic radiation exposure. Radiology 97, 653–656 (1970).
Marks, J. E. & Haus, A. G. The effect of immobilisation on localisation error in the radiotherapy of head and neck cancer. Clin. Radiol. 27, 175–177 (1976).
Kinzie, J. J., Hanks, G. E., MacLean, C. J. & Kramer, S. Patterns of care study: Hodgkin's disease relapse rates and adequacy of portals. Cancer 52, 2223–2226 (1983). This can be considered as one of the first studies to correlate misalignment of treatment beams detected by daily imaging and recurrence.
Rabinowitz, I., Broomberg, J., Goitein, M., McCarthy, K. & Leong, J. Accuracy of radiation field alignment in clinical practice. Int. J. Radiat. Oncol. Biol. Phys. 11, 1857–1867 (1985).
Byhardt, R. W., Cox, J. D., Hornburg, A. & Liermann, G. Weekly localization films and detection of field placement errors. Int. J. Radiat. Oncol. Biol. Phys. 4, 881–887 (1978).
Holloway, A. F. A localising device for a rotating cobalt therapy unit. Br. J. Radiol. 31, 227 (1958).
Johns, H. E. & Cunningham, J. R. A precision cobalt 60 unit for fixed field and rotation therapy. Am. J. Roentgenol. Radium. Ther. Nucl. Med. 81, 4–12 (1959).
Weissbluth, M., Karzmark, C. J., Steele, R. E. & Selby, A. H. The Stanford medical linear accelerator. II. Installation and physical measurements. Radiology 72, 242–253 (1959). References 52–54 illustrate some of the earlier attempts at improving image quality in the verification process of treatment by mounting X-ray devices on treatment machines. Many of more recent developments in IGRT are based on these concepts.
Verhey, L. J., Goitein, M., McNulty, P., Munzenrider, J. E. & Suit, H. D. Precise positioning of patients for radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 8, 289–294 (1982).
Leong, J. Use of digital fluoroscopy as an on-line verification device in radiation therapy. Phys. Med. Biol. 31, 985–992 (1986).
De Neve, W. et al. Routine clinical on-line portal imaging followed by immediate field adjustment using a tele-controlled patient couch. Radiother. Oncol. 24, 45–54 (1992).
Ezz, A. et al. Daily monitoring and correction of radiation field placement using a video-based portal imaging system: a pilot study. Int. J. Radiat. Oncol. Biol. Phys. 22, 159–165 (1992).
Meertens, H., Van Herk, M. & Weeda, J. A liquid ionisation detector for digital radiography of therapeutic megavoltage photon beams. Phys. Med. Biol. 30, 313–321 (1985).
Van Herk, M. & Meertens, H. A matrix ionisation chamber imaging device for on-line patient setup verification during radiotherapy. Radiother. Oncol. 11, 369–378 (1988).
Herman, M. G. et al. Clinical use of electronic portal imaging: report of AAPM Radiation Therapy Committee Task Group 58. Med. Phys. 28, 712–737 (2001). Electronic portal imaging devices initiated the concept of IGRT as “in-room imaging during the course of treatment with decisions made based on this information”. The AAPM report TG 58 offers a nice overview of the different technical solutions, clinical use and quality assurance.
Bel, A. et al. A computerized remote table control for fast on-line patient repositioning: implementation and clinical feasibility. Med. Phys. 27, 354–358 (2000).
Jaffray, D. A., Siewerdsen, J. H., Wong, J. W. & Martinez, A. A. Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 53, 1337–1349 (2002).
Verellen, D. et al. Quality assurance of a system for improved target localization and patient set-up that combines real-time infrared tracking and stereoscopic X-ray imaging. Radiother. Oncol. 67, 129–141 (2003).
Verellen, D. in Image-guided IMRT: Concepts and Clinical Applications (eds Bortfeld, T., Schmiidt-Ulrich, R. & De Neve, W.) (Springer-Verlag, Berlin, 2005).
Verellen, D., Soete, G., Linthout, N., Tournel, K. & Storme, G. Optimal control of set-up margins and internal margins for intra- and extracranial radiotherapy using stereoscopic kilovoltage imaging. Cancer Radiother. 10, 235–244 (2006).
Soete, G., Verellen, D., Tournel, K. & Storme, G. Setup accuracy of stereoscopic X-ray positioning with automated correction for rotational errors in patients treated with conformal arc radiotherapy for prostate cancer. Radiother. Oncol. 80, 371–373 (2006).
Linthout, N. et al. Assessment of secondary patient motion induced by automated couch movement during on-line 6 dimensional repositioning in prostate cancer treatment. Radiother. Oncol. 83, 168–174 (2007).
Murphy, M. J. An automatic six-degree-of-freedom image registration algorithm for image-guided frameless stereotaxic radiosurgery. Med. Phys. 24, 857–866 (1997).
Shirato, H. et al. Four-dimensional treatment planning and fluoroscopic real-time tumor tracking radiotherapy for moving tumor. Int. J. Radiat. Oncol. Biol. Phys. 48, 435–442 (2000).
Shirato, H. et al. Physical aspects of a real-time tumor-tracking system for gated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 48, 1187–1195 (2000).
Murphy, M. J. et al. The effectiveness of breath-holding to stabilize lung and pancreas tumors during radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 53, 475–482 (2002).
Verellen, D. et al. Importing measured field fluences into the treatment planning system to validate a breathing synchronized DMLC-IMRT irradiation technique. Radiother. Oncol. 78, 332–338 (2006).
Verellen, D. et al. Breathing synchronized irradiation using stereoscopic kV-imaging to limit influence of interplay between leaf motion and organ motion in 3D-CRT and IMRT: Dosimetric verification and first clinical experience. Int. J. Radiat. Oncol. Biol. Phys. 66, 108–119 (2006).
Lu, W., Parikh, P. J., Hubenschmidt, J. P., Bradley, J. D. & Low, D. A. A comparison between amplitude sorting and phase-angle sorting using external respiratory measurement for 4D CT. Med. Phys. 33, 2964–2974 (2006).
Ford, E. C., Mageras, G. S., Yorke, E. & Ling, C. C. Respiration-correlated spiral CT: a method of measuring respiratory-induced anatomic motion for radiation treatment planning. Med. Phys. 30, 88–97 (2003).
Hansen, V. N., Evans, P. M. & Swindell, W. The application of transit dosimetry to precision radiotherapy. Med. Phys. 23, 713–721 (1996).
Pasma, K. L., Heijmen, B. J., Kroonwijk, M. & Visser, A. G. Portal dose image (PDI) prediction for dosimetric treatment verification in radiotherapy. I. An algorithm for open beams. Med. Phys. 25, 830–840 (1998).
Mackie, T. R. et al. Image guidance for precise conformal radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 56, 89–105 (2003).
Lof, J., Lind, B. K. & Brahme, A. An adaptive control algorithm for optimization of intensity modulated radiotherapy considering uncertainties in beam profiles, patient set-up and internal organ motion. Phys. Med. Biol. 43, 1605–1628 (1998).
Brahme, A. Biologically optimized 3-dimensional in vivo predictive assay-based radiation therapy using positron emission tomography-computerized tomography imaging. Acta Oncol. 42, 123–136 (2003).
Song, P. Y. et al. A comparison of four patient immobilization devices in the treatment of prostate cancer patients with three dimensional conformal radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 34, 213–219 (1996).
Wulf, J., Hadinger, U., Oppitz, U., Olshausen, B. & Flentje, M. Stereotactic radiotherapy of extracranial targets: CT-simulation and accuracy of treatment in the stereotactic body frame. Radiother. Oncol. 57, 225–236 (2000).
Hodge, W. et al. Feasibility report of image guided stereotactic body radiotherapy (IG-SBRT) with tomotherapy for early stage medically inoperable lung cancer using extreme hypofractionation. Acta Oncol. 45, 890–896 (2006).
Van Herk, M., Remeijer, P. & Lebesque, J. V. Inclusion of geometric uncertainties in treatment plan evaluation. Int. J. Radiat. Oncol. Biol. Phys. 52, 1407–1422 (2002).
Zhang, L. et al. Multiple regions-of-interest analysis of setup uncertainties for head-and-neck cancer radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 64, 1559–1569 (2006).
Polat, B., Wilbert, J., Baier, K., Flentje, M. & Guckenberger, M. Nonrigid patient setup errors in the head-and-neck region. Strahlenther. Onkol. 183, 506–511 (2007).
Kashani, R., Hub, M., Kessler, M. L. & Balter, J. M. Technical note: a physical phantom for assessment of accuracy of deformable alignment algorithms. Med. Phys. 34, 2785–2788 (2007).
Murphy, M. et al. The management of imaging dose during image-guided radiotherapy: Report of the AAPM Task Group 75. Med. Phys. 34, 4041–4063. (2007).
Biggs, P. J., Goitein, M. & Russell, M. D. A diagnostic X ray field verification device for a 10 MV linear accelerator. Int. J. Radiat. Oncol. Biol. Phys. 11, 635–643 (1985).
Shiu, A. S., Hogstrom, K. R., Janjan, N. A., Fields, R. S. & Peters, L. J. Technique for verifying treatment fields using portal images with diagnostic quality. Int. J. Radiat. Oncol. Biol. Phys. 13, 1589–1594 (1987).
Munro, P. & Bouius, D. C. X-ray quantum limited portal imaging using amorphous silicon flat-panel arrays. Med. Phys. 25, 689–702 (1998).
Guckenberger, M. et al. Precision of image-guided radiotherapy (IGRT) in six degrees of freedom and limitations in clinical practice. Strahlenther. Onkol. 183, 307–313 (2007).
Murphy, M. J. et al. Image-guided radiosurgery for the spine and pancreas. Comput. Aided Surg. 5, 278–288. 2000.
Aoki, Y. et al. An integrated radiotherapy treatment system and its clinical application. Radiat. Med. 5, 131–141 (1987).
Court, L., Rosen, I., Mohan, R. & Dong, L. Evaluation of mechanical precision and alignment uncertainties for an integrated CT/LINAC system. Med. Phys. 30, 1198–1210 (2003).
Kuriyama, K. et al. A new irradiation unit constructed of self-moving gantry-CT and linac. Int. J. Radiat. Oncol. Biol. Phys. 55, 428–435 (2003).
Uematsu, M. et al. Intrafractional tumor position stability during computed tomography (CT)-guided frameless stereotactic radiation therapy for lung or liver cancers with a fusion of CT and linear accelerator (FOCAL) unit. Int. J. Radiat. Oncol. Biol. Phys. 48, 443–448 (2000).
Holupka, E. J., Kaplan, I. D., Burdette, E. C. & Svensson, G. K. Ultrasound image fusion for external beam radiotherapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 35, 975–984 (1996).
Lattanzi, J. et al. A comparison of daily CT localization to a daily ultrasound-based system in prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 43, 719–725 (1999).
Langen, K. M. et al. Evaluation of ultrasound-based prostate localization for image-guided radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 57, 635–644 (2003).
Van den Heuvel, F. et al. Independent verification of ultrasound based image-guided radiation treatment, using electronic portal imaging and implanted gold markers. Med. Phys. 30, 2878–2887 (2003).
Seiler, P. G., Blattmann, H., Kirsch, S., Muench, R. K. & Schilling, C. A novel tracking technique for the continuous precise measurement of tumour positions in conformal radiotherapy. Phys. Med. Biol. 45, N103-N110 (2000).
Litzenberg, D. W. et al. Positional stability of electromagnetic transponders used for prostate localization and continuous, real-time tracking. Int. J. Radiat. Oncol. Biol. Phys. 68, 1199–1206 (2007).
Takai, Y., Mitsuya, M. & Nemoto, K. Development of a new linear accelerator mounted with dual X-ray fluorosocpy using amorphous silicon flat panel X-ray sensors to detect a gold seed in a tumor at real treatment position. Int. J. Radiat. Oncol. Biol. Phys. 51, 381 (2001).
Pouliot, J. et al. Low-dose megavoltage cone-beam CT for radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 61, 552–560 (2005).
Mackie, T. R. et al. Tomotherapy: a new concept for the delivery of dynamic conformal radiotherapy. Med. Phys. 20, 1709–1719 (1993).
Fenwick, J. D. et al. Quality assurance of a helical tomotherapy machine. Phys. Med. Biol. 49, 2933–2953 (2004).
Carol, M. Peacock: a system for planning and rotational delivery of intensity-modulated fields. Int. J. Imag. Syst. Technol. 6, 56–61 (1995).
Verellen, D., Linthout, N., Van den, B. D., Bel, A. & Storme, G. Initial experience with intensity-modulated conformal radiation therapy for treatment of the head and neck region. Int. J. Radiat. Oncol. Biol. Phys. 39, 99–114 (1997).
Verellen, D., Linthout, N. & Storme, G. Target localization and treatment verification for intensity modulated conformal radiation therapy of the head and neck region. Strahlenther. Onkol. 174, 19–27 (1998).
Kamino, Y. et al. Development of a four-dimensional image-guided radiotherapy system with a gimbaled X-ray head. Int. J. Radiat. Oncol. Biol. Phys. 66, 271–278 (2006).
Raaijmakers, A. J., Raaymakers, B. W., van der, M. S. & Lagendijk, J. J. Integrating a MRI scanner with a 6 MV radiotherapy accelerator: impact of the surface orientation on the entrance and exit dose due to the transverse magnetic field. Phys. Med. Biol. 52, 929–939 (2007).
Janek, S., Svensson, R., Jonsson, C. & Brahme, A. Development of dose delivery verification by PET imaging of photonuclear reactions following high energy photon therapy. Phys. Med. Biol. 51, 5769–5783 (2006).
Langen, K. M. & Jones, D. T. Organ motion and its management. Int. J. Radiat. Oncol. Biol. Phys. 50, 265–278 (2001). The authors compiled and reviewed existing data on inter- and intra-fraction motion of different organs and tumours, and discussed some of the techniques that can be used to manage this motion in radiotherapy.
Lax, I., Blomgren, H., Naslund, I. & Svanstrom, R. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol. 33, 677–683 (1994).
Wong, J. W. et al. The use of active breathing control (ABC) to reduce margin for breathing motion. Int. J. Radiat. Oncol. Biol. Phys. 44, 911–919 (1999).
Mah, D. et al. Technical aspects of the deep inspiration breath-hold technique in the treatment of thoracic cancer. Int. J. Radiat. Oncol. Biol. Phys. 48, 1175–1185 (2000).
Caldwell, C. B., Mah, K., Skinner, M. & Danjoux, C. E. Can PET provide the 3D extent of tumor motion for individualized internal target volumes? A phantom study of the limitations of CT and the promise of PET. Int. J. Radiat. Oncol. Biol. Phys. 55, 1381–1393 (2003).
Ohara, K. et al. Irradiation synchronized with respiration gate. Int. J. Radiat. Oncol. Biol. Phys. 17, 853–857 (1989).
Keall, P. J., Kini, V. R., Vedam, S. S. & Mohan, R. Motion adaptive x-ray therapy: a feasibility study. Phys. Med. Biol. 46, 1–10 (2001).
Brock, K. K. et al. Inclusion of organ deformation in dose calculations. Med. Phys. 30, 290–295 (2003).
Bortfeld, T., Jiang, S. B. & Rietzel, E. Effects of motion on the total dose distribution. Semin. Radiat. Oncol. 14, 41–51 (2004).
Guckenberger, M. et al. Four-dimensional treatment planning for stereotactic body radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 69, 276–285 (2007).
Bosmans, G. et al. An “in silico” clinical trial comparing free breathing, slow and respiration correlated computed tomography in lung cancer patients. Radiother. Oncol. 81, 73–80 (2006).
Faria, S. et al. Radiotherapy volume delineation with PET in lung cancer may be less useful than foreseen. J. Thorac. Oncol. 2, 347–348 (2007).
Lagerwaard, F. J. et al. Multiple 'slow' CT scans for incorporating lung tumor mobility in radiotherapy planning. Int. J. Radiat. Oncol. Biol. Phys. 51, 932–937 (2001).
Jiang, S. B. et al. An experimental investigation on intra-fractional organ motion effects in lung IMRT treatments. Phys. Med. Biol. 48, 1773–1784 (2003).
de Mey, J. et al. Percutaneous placement of marking coils before stereotactic radiation therapy of malignant lung lesions. J. Vasc. Interv. Radiol. 16, 51–56 (2005).
Schweikard, A., Glosser, G., Bodduluri, M., Murphy, M. J. & Adler, J. R. Robotic motion compensation for respiratory movement during radiosurgery. Comput. Aided Surg. 5, 263–277 (2000).
Mageras, G. S. et al. Fluoroscopic evaluation of diaphragmatic motion reduction with a respiratory gated radiotherapy system. J. Appl. Clin. Med. Phys. 2, 191–200 (2001).
Hanley, J. et al. Deep inspiration breath-hold technique for lung tumors: the potential value of target immobilization and reduced lung density in dose escalation. Int. J. Radiat. Oncol. Biol. Phys. 45, 603–611 (1999).
Murphy, M. J. Tracking moving organs in real time. Semin. Radiat. Oncol. 14, 91–100 (2004).
Keall, P. J. et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med. Phys. 33, 3874–3900 (2006). This report describes observed magnitudes of respiratory motion, discusses specific problems related to radiotherapy, explains techniques to manage respiratory motion and gives recommendations in the applications of these techniques for patient care.
Suit, H. D. in Proc. Conf. Time Dose Relationships Radiat. Biol. Applied Radiother. (Brookhaven National Laboratory, New York, 1970).
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
National Cancer Institute
Glossary
- Gross tumour volume
-
The gross palpable or visible/demonstrable extent and location of malignant growth.
- Clinical target volume
-
A tissue volume that contains the gross tumour volume and/or subclinical malignant disease, which is to be eliminated. This volume has to be treated adequately in order to achieve the aim of radiotherapy: cure or palliation.
- Planning target volume
-
An additional margin added to the CTV that is a statistical construct to ensure that the desired dose can be anatomically realized in the CTV during treatment.
- Image-guided radiotherapy
-
Frequent imaging in the treatment room during a course of radiotherapy to guide the treatment process.
- Conformal radiotherapy
-
Describes the aim in radiotherapy of conforming or shaping the high-dose volume to the planning treatment volume. Alternatively, conformal avoidance refers to sparing of organs at risk.
- Normal-tissue complication probability
-
With the introduction of 3D treatment planning systems, it has become possible to calculate and evaluate the dose distribution not only in tumours but also in nearby normal tissues. From these 3D dose distributions it is possible to model the outcome in biological terms of tumour control probability and normal-tissue complication probability. However, proper clinical validation of these models is still lacking.
- Intensity-modulated radiotherapy
-
Radiotherapy technique in which the intensity of irradiation varies within a radiation field. This can be obtained by using differential dose absorbers or by varying the time of radiation at different points.
- Dose painting by numbers
-
Experimental intensity-modulated radiotherapy strategy in which the intensity of radiation intentionally varies within a tumour, on the basis of estimated levels of radioresistance that were assessed by biological and physical imaging modalities.
- Permanent seed implantation
-
Radiotherapy in which the radiation source is implanted into the tumour (also known as brachytherapy). Prostate brachytherapy can deliver high and concentrated doses of radiation to the prostate gland.
- Tomotherapy
-
A specially designed collimator generates an intensity-modulated profile and at the same time the gantry rotates about the long axis of the patient and as such irradiates a slice of the patient. One approach is the slice-by-slice arc rotation approach, in which the patient is translated longitudinally between consecutive gantry rotations to treat sequential transaxial slices. In the other approach, helical tomotherapy, the patient is being translated longitudinally, slowly and continuously, during the gantry rotation.
- Computed tomography
-
In radiotherapy, volumetric IGRT solutions can be based on conventional kV X-ray sources or high energy MV photon beams that are used for treatment.
- Treatment simulator
-
The treatment simulator is a machine that emulates the geometry of the treatment unit, but uses diagnostic quality X-rays to carry out localization and verification of the patient in treatment position.
- Linear accelerator
-
A device that uses high frequency electromagnetic waves to accelerate charged particles such as electrons to high energies through a linear tube. The high-energy electron beam itself can be used for treating superficial tumours or it can be made to strike a target to produce high-energy (MV) X-rays for treating deep-seated tumours.
- Image registration
-
The process of registering different image sets of the same modality (for example, CT–CT) or different modalities (for example, CT–MRI or CT–PET). This registration can be affine (that is, one set is translated, rotated or rescaled to match with the primary set assuming the patient's anatomy was not deformed) or deformable (that is, the registration algorithm deforms the secondary set to cope with internal and external deformations of anatomy).
- Electronic portal imaging device
-
A device that enables automated acquisition of images acquired with a treatment beam.
- Offline patient set-up
-
The offline approach monitors the position of the patient during a limited number of fractions and adapts the safety margins accordingly. This approach does not allow for decreasing the treatment margins sufficiently for aggressive conformal radiotherapy.
- Online patient set-up
-
The online approach offers the possibility of reducing most geometric errors (both systematic and random), but is considered to be time consuming and requires automated control of the treatment couch to make it efficient in clinical practice.
- Peripheral solutions to IGRT
-
In-room imaging systems that are not mounted physically on the treatment-delivery system.
- Isocentre
-
The point of intersection of the central axis of the radiation beam and the horizontal axis of rotation of the gantry. Traditionally the centre of the planning treatment volume coincides with the isocentre.
- On-Board IGRT system
-
In-room imaging systems that are physically attached to the treatment-delivery system.
- Cone beam volumetric CT
-
A CT scanning method in which the fan-beam and linear detector array is replaced with an open-beam and large-area flat-panel detector to generate volumetric images through a single rotation of the system.
- Dose volume histogram
-
An alternative method for displaying the results of dose calculation. The histogram shows the percentage of the volume of any structure that is irradiated above a particular dose level. More correctly known as cumulative DVH.
- Multi-leaf collimator
-
Computers have enabled the replacement of field-shaping beam blocks, which create irregularly shaped irradiation fields to spare vulnerable tissues, with an MLC. MLCs consist of 40–120 movable leaves, with a width varying between 0.2 and 1.0 cm, that are arranged in opposed pairs.
Rights and permissions
About this article
Cite this article
Verellen, D., Ridder, M., Linthout, N. et al. Innovations in image-guided radiotherapy. Nat Rev Cancer 7, 949–960 (2007). https://doi.org/10.1038/nrc2288
Issue Date:
DOI: https://doi.org/10.1038/nrc2288