Key Points
-
In many cancers, the normal process for eliminating unwanted cells (apoptosis) is deregulated.
-
The deregulation of apoptosis leads to the unchecked growth of tumours and the development of resistance to chemotherapy.
-
Drugs that restore apoptosis might selectively kill cancer cells that have triggered a death signal and have become dependent on the deregulation of apoptosis pathways.
-
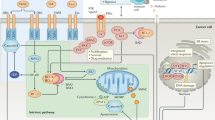
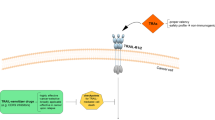
Agonistic antibodies against the tumour-necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) receptors and a soluble, truncated TRAIL ligand are in phase I/II clinical trials for the treatment of cancer.
-
BCL2 antisense oligonucleotides are in phase III clinical trials and pre-registration, and small-molecule BCL2-family inhibitors are in early phase I clinical trials and in late preclinical discovery for the treatment of chronic lymphocytic leukaemia and solid tumours.
-
IAP (inhibitor of apoptosis) protein inhibitors, MDM2 antagonists and other apoptosis-inducing compounds are under preclinical examination for possible use in cancer therapy.
-
For these compounds to succeed, it will be important to test them in well-designed clinical trials to determine the cancer patients that are most likely to respond to the particular agent, the optimum dose and schedule, and the best combination with other drugs.
Abstract
Apoptosis is deregulated in many cancers, making it difficult to kill tumours. Drugs that restore the normal apoptotic pathways have the potential for effectively treating cancers that depend on aberrations of the apoptotic pathway to stay alive. Apoptosis targets that are currently being explored for cancer drug discovery include the tumour-necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) receptors, the BCL2 family of anti-apoptotic proteins, inhibitor of apoptosis (IAP) proteins and MDM2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Thompson, C. B. Apoptosis in the pathogenesis and treatment of disease. Science 267, 1456–1462 (1995).
Danial, N. N. & Korsmeyer, S. J. Cell death: critical control points. Cell 116, 205–219 (2004).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Nicholson, D. W. From bench to clinic with apoptosis-based therapeutic agents. Nature 407, 810–816 (2000).
Nagata, S. Apoptosis by death factor. Cell 88, 355–365 (1997).
Budihardjo, I., Oliver, H., Lutter, M., Luo, X. & Wang, X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 15, 269–290 (1999).
Baell, J. B. & Huang, D. C. S. Prospects for targeting the Bcl-2 family of proteins to develop novel cytotoxic drugs. Biochem. Pharmacol. 64, 851–863 (2002).
Deveraux, Q. L., Takahashi, R., Salvesen, G. S. & Reed, J. C. X-linked IAP is a direct inhibitor of cell-death proteases. Nature 388, 300–304 (1997).
Du, C., Fang, M., Li, Y., Li, L. & Wang, X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102, 33–42 (2000).
Verhagen, A. M. et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102, 43–53 (2000).
Chene, P. Inhibiting the p53–MDM2 interaction: an important target for cancer therapy. Nature Rev. Cancer 3, 102–109 (2003).
Ashkenazi, A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nature Rev. Cancer 2, 420–421 (2002).
Hymowitz, S. G. et al. Triggering cell death: the crystal structure of Apo2L/TRAIL in a complex with death receptor 5. Mol. Cell 4, 563–571 (1999).
Mongkolsapaya, J. et al. Structure of the TRAIL–DR5 complex reveals mechanisms conferring specificity in apoptotis initiation. Nature Struct. Biol. 6, 1048–1053 (1999).
Cha, S. S. et al. Crystal structure of TRAIL–DR5 complex identifies a critical role of the unique frame insertion in conferring recognition specificity. J. Biol. Chem. 275, 31171–31177 (2000).
Kelley, S. K. & Ashkenazi, A. Targeting death receptors in cancer with Apo2L/TRAIL. Curr. Opin. Pharmacol. 4, 333–339 (2004). A comprehensive review on targeting TRAIL receptors.
Chuntharapai, A. et al. Isotype-dependent inhibition of tumor growth in vivo by monoclonal antibodies to death receptor 4. J. Immunol. 166, 4891–4898 (2001).
Ichikawa, K. et al. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nature Med. 7, 954–960 (2001).
Takeda, K. et al. Induction of tumor-specific T cell immunity by anti-DR5 antibody therapy. J. Exp. Med. 199, 437–448 (2004).
Presta, L. G. Engineering antibodies for therapy. Curr. Pharm. Biotechnol. 3, 237–256 (2002).
Shankar, S., Chen, X. & Srivastava, R. K. Effects of sequential treatments with chemotherapeutic drugs followed by TRAIL on prostate cancer in vitro and in vivo. Prostate 62, 165–186 (2005).
Inoue et al. Histone deacetylase inhibitors potentiate TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in lymphoid malignancies. Cell Death Differ. 11, S193–S206 (2004).
Naka, T. et al. Effects of tumor necrosis factor-related apoptosis-inducing ligand alone and in combination with chemotherapeutic agents on patients' colon tumors grown in SCID mice. Cancer Res. 62, 5800–5806 (2002).
Pollack, I. F., Erff, M. & Ashkenazi, A. Direct stimulation of apoptotic signaling by soluble Apo2L/tumor necrosis factor-related apoptosis-inducing ligand leads to selective killing of glioma cells. Clin. Cancer Res. 7, 1362–1369 (2001).
Jin, H. et al. Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand cooperates with chemotherapy to inhibit orthotopic lung tumor growth and improve survival. Cancer Res. 64, 4900–4905 (2004).
Ray, S. & Almasan, A. Apoptosis induction in prostate cancer cells and xenografts by combined treatment with Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand and CPT-11. Cancer Res. 63, 4713–4723 (2003).
Mitsiades, C. S. et al. TRAIL/Apo2L ligand selectively induces apoptosis and overcomes drug resistance in multiple myeloma: therapeutic applications. Blood 98, 795–804 (2001).
Chinnaiyan, A. M. et al. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc. Natl Acad. Sci USA 97, 1754–1759 (2000).
Ashkenazi, A. et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Invest. 104, 155–162 (1999).
Kelley, S. K. et al. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J. Pharmacol. Exp. Ther. 299, 31–38 (2001).
LeBlanc, H. N. & Ashkenazi, A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 10, 66–75 (2003).
Jin, Z., McDonald III, E. R., Dicker, D. T. & El-Deiry, W. S. Deficient tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor transport to the cell surface in human colon cancer cells selected for resistance to TRAIL-induced apoptosis. J. Biol. Chem. 279, 35829–35839 (2004).
Kazhdan, I. & Marciniak, R. A. Death receptor 4 (DR4) efficiently kills breast cancer cells irrespective of their sensitivity to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Cancer Gene Ther. 11, 691–698 (2004).
Chawla-Sarkar, M. et al. Downregulation of Bcl-2, FLIP or IAPs (XIAP and survivin) by siRNAs sensitizes resistant melanoma cells to Apo2L/TRAIL-induced apoptosis. Cell Death Differ. 11, 915–923 (2004).
Fulda, S., Wick, W., Weller, M. & Debatin, K. M. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nature Med. 8, 808–815 (2002).
LeBlanc, H. et al. Tumor-cell resistance to death receptor-induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nature Med. 8, 274–281 (2002).
Deng, Y., Lin, Y. & Wu, X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 16, 33–45 (2002).
Ravi, R. & Bedi, A. Requirement of BAX for TRAIL/Apo2L-induced apoptosis of colorectal cancers: synergism with sulindac-mediated inhibition of Bcl-xL. Cancer Res. 62, 1583–1587 (2002).
Kelekar, A. & Thompson, C. B. Bcl-2 family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 8, 324–330 (1998).
Huang, D. C. & Strasser, A. BH3-only proteins-essential initiators of apoptotic cell death. Cell 103, 839–842 (2000).
Wei, M. C. et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 (2001).
Petros, A. M., Olejniczak, E. T. & Fesik, S. W. Structural biology of the Bcl-2 family of proteins. Biochim. Biophys. Acta 1644, 83–94 (2004).
Sattler, M. et al. Structure of Bcl-xL–Bak peptide complex: recognition between regulators of apoptosis. Science 275, 983–986 (1997). First structure that defined pro- and anti-apoptotic BCL2 family members interacting with one another.
Petros, A. M. et al. Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis, and biophysical studies. Protein Sci. 9, 2528–2534 (2000).
Kirkin, V., Joos, S. & Zornig, M. The role of Bcl-2 family members in tumorigenesis. Biochim. Biophys. Acta 1644, 229–249 (2004).
Tsujimoto, Y., Finger, L. R., Yunis, J., Nowell, P. C. & Croce, C. M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science 226, 1097–1099 (1984).
Tsujimoto, Y., Gorham, J., Cossman, J., Jaffe, E. & Croce, C. M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science 229, 1390–1393 (1985).
Gaulard, P. et al. Expression of the bcl-2 gene product in follicular lymphoma. Am. J. Pathol. 140, 1089–1095 (1992).
Ben-Ezra, J. M., Kornstein, M. J., Grimes, M. M. & Krysal, G. Small cell carcinomas of the lung express the Bcl-2 protein. Am. J. Pathol. 145, 1036–1040 (1994).
Higashiyama, M., Doi, O., Kodama, K., Yokouchi, H. & Tateishi, R. High prevalence of bcl-2 oncoprotein expression in small cell lung cancer. Anticancer Res. 15, 503–505 (1995).
Schena, M. et al. Growth- and differentiation-associated expression of bcl-2 in B-chronic lymphocytic leukemia cells. Blood 79, 2981–2989 (1992).
Harada, N. et al. Expression of Bcl-2 family of proteins in fresh myeloma cells. Leukemia 12, 1817–1820 (1998).
Leiter, U., Schmid, R. M., Kaskel, P., Peter, R. U. & Krahn, G. Antiapoptotic bcl-2 and bcl-xL in advanced malignant melanoma. Arch. Dermatol. Res. 292, 225–232 (2000).
Matsushima, H. et al. Combined analysis with Bcl-2 and p53 immunostaining predicts poorer prognosis in prostatic carcinoma. J. Urol. 158, 2278–2283 (1997).
Keshgegian, A. A., Johnston, E. & Cnaan, A. Bcl-2 oncoprotein positivity and high MIB-1 (Ki-67) proliferative rate are independent predictive markers for recurrence in prostate carcinoma. Am. J. Clin. Pathol. 110, 443–449 (1998).
Mano, Y. et al. Bcl-2 as a predictor of chemosensitivity and prognosis in primary epithelial ovarian cancer. Eur. J. Cancer 35, 1214–1219 (1999).
Rajkumar, T. et al. Prognostic significance of Bcl-2 and p53 protein expression in stage IIB and IIIB squamous cell carcinoma of the cervix. Eur. J. Gynaecol. Oncol. 19, 556–560 (1998).
Ye, D. et al. Bcl-2/bax expression and p53 gene status in human bladder cancer: relationship to early recurrence with intravesical chemotherapy after resection. J. Urol. 160, 2025–2028 (1998).
Nakata, B. et al. Predictive value of Bcl-2 and Bax protein expression for chemotherapeutic effect in gastric cancer. A pilot study. Oncology 55, 543–547 (1998).
Fries, H. et al. Moderate activation of the apoptosis inhibitor bcl-xL worsens the prognosis in pancreatic cancer. Ann. Surg. 228, 780–787 (1998).
Lipponen, P. et al. Apoptosis suppressing protein bcl-2 is expressed in well-differentiated breast carcinomas with favourable prognosis. J. Pathol. 177, 49–55 (1995).
Le, M. G. et al. c-myc, p53 and bcl-2, apoptosis-related genes in infiltrating breast carcinomas: evidence of a link between bcl-2 protein over-expression and a lower risk of metastasis and death in operable patients. Int. J. Cancer 84, 562–567 (1999).
Nakopoulou, L. et al. bcl-2 protein expression is associated with a prognostically favourable phenotype in breast cancer irrespective of p53 immunostaining. Histopathology 34, 310–319 (1999).
Sinicrope, F. A., Hart, J., Michelassi, F. & Lee, J. J. Prognostic value of bcl-2 oncoprotein expression in stage II colon carcinoma. Clin. Cancer Res. 1, 1103–1110 (1995).
Ofner, D. et al. Immunohistochemically detectable bcl-2 expression in colorectal carcinoma: correlation with tumour stage and patient survival. Br. J. Cancer 72, 981–985 (1995).
Baretton, G. B. et al. Apoptosis and immunohistochemical bcl-2 expression in colorectal adenomas and carcinomas. Aspects of carcinogenesis and prognostic significance. Cancer 77, 255–264 (1996).
Leahy, D. T., Mulcahy, H. E., O'Donoghue, D. P. & Parfrey, N. A. bcl-2 protein expression is associated with better prognosis in colorectal cancer. Histopathology 35, 360–367 (1999).
Ohmori, T. et al. Apoptosis of lung cancer cells caused by some anti-cancer agents (MMC, CPT-11, ADM) is inhibited by bcl-2. Biochem. Biophys. Res. Commun. 192, 30–36 (1993).
Minn, A. J., Rudin, C. M., Boise, L. H. & Thompson, C. B. Expression of bcl-xL can confer a multidrug resistance phenotype. Blood 86, 1903–1910 (1995).
Harima, Y. et al. Bax and Bcl-2 expressions predict response to radiotherapy in human cervical cancer. J. Cancer Res. Clin. Oncol. 124, 503–510 (1998).
Mackey, T. J., Borkowski, A., Amin, P., Jacobs, S. C. & Kyprianou, N. bcl-2/bax ratio as a predictive marker for therapeutic response to radiotherapy in patients with prostate cancer. Urology 52, 1085–1090 (1998).
Amundson, S. A. et al. An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 60, 6101–6110 (2000). Support for BCL-X L as a cancer target.
Klasa, R. J., Gillum, A. M., Klem, R. E. & Frankel, S. R. Oblimersen Bcl-2 antisense: facilitating apoptosis in anticancer treatment. Antisense Nucleic Acid Drug Dev. 12, 193–213 (2002). Summary of the use of BCL2 antisense in anticancer treatment.
Cummings, J., Ward, T. H., Ranson, M. & Dive, C. Apoptosis pathway-targeted drugs — from the bench to the clinic. Biochim. Biophys. Acta 1705, 53–66 (2004).
Gleave, M. E. & Monia, B. P. Antisense therapy for cancer. Nature Rev. Cancer 5, 468–479 (2005).
Frantz, S. Lessons learnt from Genasense's failure. Nature Rev. Drug Discov. 3, 542–543 (2004).
Agrawal, S. & Kandimalla, E. R. Antisense and/or immunostimulatory oligonucleotide therapeutics. Curr. Cancer Drug Targets 1, 197–209 (2001).
Zangemeister-Wittke, U. et al. A novel bispecific antisense oligonucleotide inhibiting both bcl-2 and bcl-XL expression efficiently induces apoptosis in tumor cells. Clin. Cancer Res. 6, 2547–2555 (2000).
Holinger, E. P., Chittenden, T. & Lutz, R. J. Bak BH3 peptides antagonize Bcl-xL function and induce apoptosis through cytochrome c-independent activation of caspases. J. Biol. Chem. 274, 13298–13304 (1999).
Wang, J. L. et al. Cell permeable Bcl-2 binding peptides: a chemical approach to apoptosis induction in tumor cells. Cancer Res. 60, 1498–1505 (2000).
Walensky, L. D. et al. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science 305, 1466–1470 (2004).
Kutzki, O. et al. Development of a potent Bcl-xL antagonist based on α-helix mimicry. J. Am. Chem. Soc. 124, 11838–11839 (2002).
Tzung, S. P. et al. Antimycin A mimics a cell-death-inducing Bcl-2 homology domain 3. Nature Cell Biol. 3, 183–191 (2001).
Chan, S. L. et al. Identification of chelerythrine as an inhibitor of BclXL function. J. Biol. Chem. 278, 20453–20456 (2003).
Real, P. J. et al. Breast cancer cells can evade apoptosis-mediated selective killing by a novel small molecule inhibitor of Bcl-2. Cancer Res. 64, 7947–7953 (2004).
Wang, J. L. et al. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc. Natl Acad. Sci. USA 97, 7124–7129 (2000).
Kitada, S. et al. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J. Med. Chem. 46, 4259–4264 (2003).
Mohammad, R. M. et al. Preclinical studies of a nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-X(L) [(–)-gossypol] against diffuse large cell lymphoma. Mol. Cancer Ther. 4, 13–21 (2005).
Oliver, C. L. et al. (–)-Gossypol acts directly on the mitochondria to overcome Bcl-2 and Bcl-X(L)-mediated apoptosis resistance. Mol. Cancer Ther. 4, 23–31 (2005).
Degterev, A. et al. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xL. Nature Cell Biol. 3, 173–182 (2001).
Enyedy, I. J. et al. Discovery of small-molecule inhibitors of Bcl-2 through structure-based computer screening. J. Med. Chem. 44, 4313–4324 (2001).
Becattini, B. et al. Rational design and real time, in-cell detection of the proapoptotic activity of a novel compound targeting Bcl-xL. Chem. Biol. 11, 389–395 (2004).
Oltersdorf, T. et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435, 677–681 (2005). Discovery of the most potent BCL2-family inhibitor to date.
Sun, C. et al. NMR structure and mutagenesis of the inhibitor-of-apoptosis protein XIAP. Nature 401, 818–822 (1999).
Takahashi, R. et al. A single BIR domain of XIAP sufficient for inhibiting caspases. J. Biol. Chem. 273, 7787–7790 (1998).
Riedl, S. J. et al. Structural basis for the inhibition of caspase-3 by XIAP. Cell 104, 791–800 (2001).
Sun, C. et al. NMR structure and mutagenesis of the third bir domain of the inhibitor of apoptosis protein XIAP. J. Biol. Chem. 275, 33777–33781 (2000).
Shiozaki, E. N. et al. Mechanism of XIAP-mediated inhibition of caspase-9. Mol. Cell 11, 519–527 (2003).
Liu, Z. et al. Structural basis for binding of smac/DIABLO to the XIAP bir3 domain. Nature 408, 1004–1008 (2000). This reference, with reference 100, describes the structure of the SMAC–XIAP-BIR3 complex used in the design of XIAP antagonists.
Wu, G. et al. Structural basis of IAP recognition by Smac/DIABLO. Nature 408, 1008–1012 (2000).
LaCasse, E. C., Baird, S., Korneluk, R. G. & MacKenzie, A. E. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene 17, 3247–3259 (1998).
Hu, Y. et al. Antisense oligonucleotides targeting XIAP induce apoptosis and enhance chemotherapeutic activity against human lung cancer c in vitro and in vivo. Clin. Cancer Res. 9, 2826–2836 (2003).
Arnt, C. R., Chiorean, M. V., Heldebrant, M. P. Gores, G. J. & Kaufmann, S. H. Synthetic smac/DIABLO peptides enhance the effects of chemotherapeutic agents by binding XIAP and cIAP1 in situ. J. Biol. Chem. 277, 44236–44243 (2002).
Yang, L. et al. Predominant suppression of apoptosome by inhibitor of apoptosis protein in non-small cell lung cancer H460 cells: therapeutic effect of a novel polyarginine-conjugated smac peptide. Cancer Res. 63, 831–837 (2003).
Sun, H. et al. Structure-based design, synthesis, and evaluation of conformationally constrained mimetics of the second mitochondria-derived activator of caspase that target the X-linked inhibitor of apoptosis protein/caspase-9 interaction site. J. Med. Chem. 47, 4147–4150 (2004).
Sun, H. et al. Structure-based design of potent, conformationally constrained Smac mimetics. J. Am. Chem. Soc. 126, 16686–16687 (2004).
Sun, H. et al. Structure-based design, synthesis and biochemical testing of novel and potent Smac peptido-mimetics. Bioorg. Med. Chem. Lett. 15, 793–797 (2005).
Oost, T. et al. Discovery of potent antagonists of the antiapoptotic protein XIAP for the treatment of cancer. J. Med. Chem. 47, 4417–4426 (2004).
Wu, T. Y. H., Wagner, K. W., Bursulaya, B., Schultz, P. G. & Deveraux, Q. L. Development and characterization of nonpeptidic small molecule inhibitors of the XIAP/caspase-3 interaction. Chem. Biol. 10, 759–767 (2003).
Nikolovska-Coleska, Z. et al. Discovery of embelin as a cell-permeable, small-molecular weight inhibitor of XIAP through structure-based computational screening of a traditional herbal medicine three-dimensional structure database. J. Med. Chem. 47, 2430–2440 (2004).
Li, L. et al. A small molecule smac mimic potentiates TRAIL- and TNFα-mediated cell death. Science 305, 1471–1474 (2004).
Park, C. et al. Non-peptidic small molecule inhibitors of XIAP. Bioorg. Med. Chem. Lett. 15, 771–775 (2005).
Schimmer, A. D. et al. Small-molecule antagonists of apoptosis suppressor XIAP exhibit broad antitumor activity. Cancer Cell 5, 25–35 (2004).
Ambrosini, G., Adida, C. & Altieri, D. C. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nature Med. 3, 917–921 (1997). The first report of survivin.
Altieri, D. C. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene 22, 8581–8589 (2003).
Olie, R. A. et al. A novel antisense oligonucleotide targeting survivin expression induces apoptosis and sensitizes lung cancer cells to chemotherapy. Cancer Res. 60, 2805–2809 (2000).
Grossman, D., Kim, P. J., Schechner, J. S. & Altieri, D. C. Inhibition of melanoma tumor growth in vivo by survivin target. Proc. Natl Acad. Sci. USA 98, 635–640 (2001).
Momand, J., Wu, H. H. & Dasgupta, G. MDM2 — master regulator of the p53 tumor suppressor protein. Gene 242, 15–29 (2000).
Lane, D. P. & Lain, S. Therapeutic exploitation of the p53 pathway. Trends Mol. Med. 8 (Suppl.), S38–S42 (2002).
Kussie, P. H. et al. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science 274, 948–953 (1996). Structure of the p53–MDM2 complex used in the design of MDM2 inhibitors.
Bottger, A. et al. Molecular characterization of the hdm2-p53 interaction. J. Mol. Biol. 269, 744–756 (1997).
Stoll, R. et al. Chalcone derivatives antagonize interactions between the human oncoprotein MDM2 and p53. Biochemistry 2, 336–344 (2001).
Zhao, J. et al. The initial evaluation of non-peptidic small-molecule HDM2 inhibitors based on p53-HDM2 complex structure. Cancer Lett. 183, 69–77 (2002).
Duncan, S. J. et al. Isolation and structure elucidation of chlorofusin, a novel p53–MDM2 antagonist from a Fusarium sp. J. Med. Chem. Soc. 123, 554–560 (2001).
Galatin, P. S. & Abraham, D. J. A nonpeptidic sulfonamide inhibits the p53–mdm2 interaction and activates p53-dependent transcription in mdm2-overexpressing cells. J. Med. Chem. 47, 4163–4165 (2004).
Parks D. J. et al. 1,4–Benzodiazepine–2,5-diones as small molecule antagonists of the HDM2–p53 interaction: discovery and SAR. Bioorg. Med. Chem. Lett. 15, 765–770 (2005).
Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004). Discovery of the most potent MDM2 inhibitor to date.
Workman, P. Inhibiting the phosphoinositide 3-kinase pathway for cancer treatment. Biochem. Soc. Trans. 32, 393–396 (2004).
Datta, S. R. et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91, 231–241 (1997).
del Paso, L., Gonzalez-Garcia, M., Page, C., Herrera, R. & Nunez, G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278, 687–689 (1997).
Blume-Jensen, P., Janknecht, R. & Hunter, T. The kit receptor promotes cell survival via activation of PI 3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136. Curr. Biol. 8, 779–782 (1998).
Kim, A. H., Khursigara, G., Sun, X., Franke, T. F. & Chao, M. V. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase Mol. Cell. Biol. 21, 893–901 (2001).
Brunet, A. et al. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 96, 857–868 (1999).
Kops, G. J. & Burgering, B. M. Forkhead transcription factors: new insights into protein kinase B (c-akt) signaling. J. Mol. Med. 77, 656–665 (1999).
Kops, G. J. et al. Direct control of the forkhead transcription factor AFX by protein kinase B. Nature 398, 630–634 (1999).
Gelfanov, V. M. et al. Transformation of interleukin-3-dependent cells without participation of Stat5/bcl-xL: cooperation of akt with raf/erk leads to p65 nuclear factor κB-mediated antiapoptosis involving c-IAP2. Blood 98, 2508–2517 (2001).
Trencia, A. et al. Protein kinase B/Akt binds and phosphorylates PED/PEA-15, stabilizing its antiapoptotic action. Mol. Cell. Biol. 23, 4511–4521 (2003).
Mitsiades, C. S., Mitsiades, N. & Koutsilieris, M. The akt pathway: molecular targets for anti-cancer drug development. Curr. Cancer Drug Targets 4, 235–256 (2004).
Lu, Y., Wang, H. & Mills, G. B. Targeting PI3K–akt pathway for cancer therapy. Rev. Clin. Exp. Hematol. 7, 205–228 (2003).
Luo, Y. et al. Potent and selective inhibitors of akt kinases slow the progression of tumors in vivo. Mol. Cancer Therap. 4, 977–986 (2005).
Rowinsky, E. K. Targeting the molecular target of rapamycin (mTOR). Curr. Opin. Oncol. 16, 564–575 (2004).
Adams, J. The proteasome: a suitable antineoplastic target. Nature Rev. Cancer 4, 349–360 (2004).
Fribley, A., Zeng, Q. & Wang, C. Y. Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinomous cells. Mol. Cell. Biol. 24, 9696–9704 (2004).
Burke, J. R. Targeting I kappa B kinase for the treatment of inflammatory and other disorders. Curr. Opin. Drug Discov. Devel. 6, 720–728 (2003).
Chen, G. -Q. et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RARα/PML proteins. Blood 88, 1052–1061 (1996).
Acknowledgements
The author wishes to thank A. Petros and E. Olejniczak for help in preparing the figures.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
Stephen Fesik is an employee of Abbott Laboratories.
Glossary
- ANTIBODY-DEPENDENT CELLULAR CYTOTOXICITY
-
Immune cells interact with the Fc region of antibodies that are bound to a target cell through Fc receptors on their surface to mediate cell killing.
- COMPLEMENT-DEPENDENT CYTOTOXICITY
-
Antibodies can kill target cells by binding the various components of complement. When combined, the components form pores in the cell membrane, which leads to phagocytosis or lysis.
- ANTENNAPEDIA HOMEOPROTEIN
-
Cationic peptides derived from this protein can be attached to molecules to allow their transport into cells.
- α, αDI-SUBSTITUTED UNNATURAL AMINO-ACIDS
-
Amino-acids with two additional chemical groups attached to the α-position.
- TERPHENYL SCAFFOLD
-
A synthetic framework containing three phenyl rings that can be used to stabilize α-helical proteins and disrupt protein–protein interactions.
- HETEROCYCLES
-
Ring compounds with both carbon atoms and atoms of other elements in the ring.
- CHALCONE DERIVATIVES
-
1,3-diphenyl-2-propen-1-one analogues that are derived from ketones and are important intermediates in the synthesis of flavonoids. They are used to treat several diseases, including cancer.
Rights and permissions
About this article
Cite this article
Fesik, S. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer 5, 876–885 (2005). https://doi.org/10.1038/nrc1736
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc1736