Key Points
-
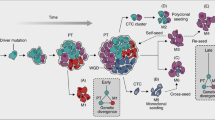
In some solid tumours, such as those of the head and neck, the presence of lymph-node metastases is closely linked to the development of distant metastases. In others, such as breast cancer, this association is less pronounced.
-
Gene-expression profiling studies of breast cancer cells indicate that specific molecular pathways are associated with haematogenous dissemination of primary tumour cells, whereas these pathways were not involved with lymphatic dissemination.
-
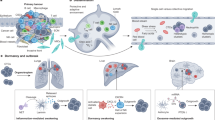
Disseminated tumour cells found in the bone marrow of patients with various types of solid tumours (for example, breast, colon and lung tumours) can be detected by sensitive immunocytochemical and molecular assays.
-
The presence of disseminated tumour cells in bone marrow predicts the development of overt metastases — both in the bone and other organs.
-
The genetic characterization of single disseminated tumour cells isolated from the bone marrow, along with gene-expression profiling studies of primary tumour cells, indicate that haematogenous dissemination is often a very early event in tumour progression. The cells seem to first disseminate from the early primary lesions and then acquire additional genetic defects.
-
Single disseminated tumour cells in the blood and bone marrow are targets for adjuvant therapy. These cells often show different properties to cells of the primary tumour, so further molecular analysis will provide additional information and will help to develop antimetastatic therapies.
Abstract
Despite recent progress in gene-expression profiling studies, the biology underlying the various patterns of metastasis that are observed in different tumour types remains unclear. The detection and characterization of disseminated tumour cells in patients with cancer has provided important new information about the cascade of metastatic events. This information has important implications for cancer prognosis and for therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fidler, I. J. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nature Rev. Cancer 3, 453–458 (2003).
Van 't Veer, L. J. et al. Gene-expression profiling predicts clinical outcome of breast cancer. Nature 415, 530–536 (2002). Shows how a specific gene-expression profile of primary breast cancers is associated with the development of metastasis and, therefore, with poor clinical outcome.
Van de Vijver, M. J. et al. A gene expression signature as a predictor for survival in breast cancer. N. Engl. J. Med. 347, 1999–2009 (2002).
Bernards, R. & Weinberg, R. A. A progression puzzle. Nature 418, 823–824 (2002). Proposes that the potential for metastasis is determined early in tumorigenesis, which explains why most cells in a primary tumour express the molecular signature that is associated with metastatic tumours. This model challenges the traditional model (see reference 15).
Pantel, K., Cote, R. & Fodstad, O. Detection and clinical relevance of micrometastatic disease. J. Natl. Cancer Inst. 91, 1113–1124 (1999).
Gath, H. & Brakenhoff, R. H. Minimal residual disease in head and neck cancer. Cancer Metast. Rev. 18, 109–126 (1999).
Kang, Y. et al. A multigenic program mediating breast cancer metastasis. Cancer Cell 3, 537–549 (2003).
Chambers, A., Groom, A. C. & MacDonald, I. C. Dissemination and growth of cancer cells in metastatic sites. Nature Rev. Cancer 2, 563–572 (2002).
Leemans, C. R., Tiwari, R., Nauta, J. J., van der Waal, I. & Snow, G. B. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer 71, 452–456 (1993).
Nieuwenhuis, E. J. C. et al. Assessment of micrometastases in lymph nodes of head and neck cancer patients by E48 Q-RT-PCR. Lab. Invest. 83, 1233–1240 (2003).
Braun, S. et al. Cytokeratin-positive bone marrow micrometastases and survival of breast cancer patients with stage I–III disease. N. Engl. J. Med. 342, 525–533 (2000). Demonstrates the independent prognostic value of detection of disseminated tumour cells in the bone marrow of patients with breast cancer, compared to a large number of controls.
Wölfle, U. et al. Molecular signature associated with micrometastasis in human breast cancer. Cancer Res. 63, 5679–5684 (2003). Shows that early systemic dissemination of breast cancer cells is associated with a specific expression signature, and that the molecular pathways associated with primary haematogenous spread or lymphatic dissemination are unrelated.
Perou, C. M. et al. Molecular portraits of human breast cancer. Nature 406, 747–752 (2000).
Ramaswamy, S., Ross, K. N., Lander, E. S. & Golub, T. R. A molecular signature of metastasis in primary solid tumors. Nature Genet. 33, 49–54 (2003).
Fidler, I. J. & Kripke, M. L. Metastasis results from pre-existing variant cells within a malignant tumour. Science 197, 893–895 (1977). Provides important experimental data to establish the traditional model of metastasis — that overt metastases are derived from small subpopulations that arise late in tumour progression.
Malumbres, M. & Barbacid, M. RAS oncogenes: the first 30 years. Nature Rev. Cancer 3, 459–465 (2003).
Harris, A. L. Hypoxia — a key regulatory factor in tumour growth. Nature Rev. Cancer 2, 38–47 (2002).
Braun, S. et al. Comparative analysis of micrometastasis to the bone marrow and lymph nodes of node-negative breast cancer patients receiving no adjuvant therapy. J. Clin. Oncol. 19, 1468–1475 (2001).
Sidransky, D. Nucleic acid-based methods for the detection of cancer. Science 278, 1054–1059 (1997).
Zippelius, A. et al. Limitations of reverse-transcriptase polymerase chain reaction analyses for detection of micrometastatic epithelial cancer cells in bone marrow. J. Clin. Oncol. 15, 2701–2708 (1997).
Bostick, P. J. et al. Limitations of specific reverse-transcriptase polymerase chain reaction markers in the detection of metastases in the lymph nodes and blood of breast cancer patients. J. Clin. Oncol. 16, 2632–2640 (1998).
Van Houten, V. M. M. et al. Molecular assays for the detection of minimal residual head and neck cancer: methods, reliability, pitfalls and solutions. Clin. Cancer Res. 6, 3803–3816 (2000).
Lindemann, F., Schlimok, G., Dirschedl, P., Witte, J. & Riethmüller, G. Prognostic significance of micrometastatic tumour cells in bone marrow of colorectal cancer patients. Lancet 340, 685–689 (1992).
Pantel, K. et al. Frequency and prognostic significance of isolated tumour cells in bone marrow of patients with non-small-cell lung cancer without overt metastases. Lancet 347, 649–653 (1996).
Janni, W. et al. The fate and prognostic value of occult metastatic cells in the bone marrow of patients with breast carcinoma between primary treatment and recurrence. Cancer 92, 46–53 (2001).
Heiss, M. M. et al. Individual development and uPA-receptor expression of disseminated tumour cells in bone marrow: a reference to early systemic disease in solid cancer. Nature Med. 1, 1035–1039 (1995).
Pierga, J. -Y. et al. Clinical significance of immunocytochemical detection of tumor cells using digital microscopy in peripheral blood and bone marrow of breast cancer patients. Clin. Cancer Res. 10, 1392–1400 (2004).
Braun, S. et al. ErbB2 over-expression on occult metastatic cells in bone marrow predicts poor clinical outcome of stage I–III breast cancer patients. Cancer Res. 61, 1890–1895 (2001).
Solakoglu, O. et al. Heterogeneous proliferative potential of occult metastatic cells in bone marrow of patients with solid epithelial tumors. Proc. Natl Acad. Sci. USA 99, 2246–2251 (2002).
Klein, C. A. et al. Genetic heterogeneity of single disseminated tumors cells in minimal residual cancer. Lancet 360, 683–689 (2002).
Schmidt-Kittler, O. et al. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc. Natl Acad. Sci. USA 100, 7737–7742 (2003).
Offner, S. et al. p53 gene mutations are not required for early dissemination of cancer cells. Proc. Natl Acad. Sci. USA 96, 6942–6946 (1999). References 29–32 provided the first data on the genetic characterization of disseminated tumour cells from the bone marrow of patients with solid tumours. These reports show that disseminated tumour cells in patients with early-stage cancer are genetically heterogeneous and have different genetic alterations to primary tumour cells.
Gray, J. W. Evidence emerges for early metastasis and parallel evolution of primary and metastatic tumors. Cancer Cell 4, 4–6 (2003).
Kuukasjarvi, T. et al. Genetic heterogeneity and clonal evolution underlying development of asynchronous metastasis in human breast cancer. Cancer Res. 57, 1597–1604 (1997).
Pierga, J. -Y. et al. Clinical significance of proliferative potential of occult metastatic cells in bone marrow of patients with breast cancer. Br. J. Cancer 89, 539–545 (2003).
Gangnus, R., Langer, S., Breit, E., Pantel, K. & Speicher, M. R. Genomic profiling of viable and proliferative micrometastatic cells from early stage breast cancer patients. Clin. Cancer Res. (in the press).
Putz, E. et al. Phenotypic characteristics of cell lines derived from disseminated cancer cells in bone marrow of patients with solid epithelial tumors: establishment of working models for human micrometastases. Cancer Res 59, 241–248 (1999).
Kraus, J., Pantel, K., Pinkel, D., Albertson, D. G. & Speicher, M. R. High-resolution genomic profiling of occult micrometastatic tumor cells. Genes Chromosom. Cancer 36, 159–166 (2003).
Luzzi, K. J. et al. Nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 153, 865–873 (1998).
Krag, D. N. & Single, R. M. Breast cancer survival according to numbers of nodes removed. Ann. Surg. Oncol. 10, 1152–1159 (2003).
Goldhirsch, A. et al. Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J. Clin. Oncol. 21, 3357–3365 (2003).
Pantel, K. et al. Differential expression of proliferation-associated molecules in individual micrometastatic carcinoma cells. J. Natl Cancer Inst. 85, 1419–1424 (1993).
Braun, S. et al. Lack of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. J. Clin. Oncol. 18, 80–86 (2000).
Albertson, D. G., Collins, C., McCormick, F. & Gray, J. W. Chromosome aberrations in solid tumors. Nature Genet. 34, 369–376 (2003).
Wiedswang, G. et al. Detection of isolated tumor cells in bone marrow is an independent prognostic factor in breast cancer. J. Clin. Oncol. 21, 3469–3478 (2003).
Pantel, K. et al. Detection and clinical implications of early systemic tumor cell dissemination in breast cancer. Clin. Cancer Res. 9, 6326–6334 (2003).
Reimers, N. et al. Role of EMMPRIN in the progression of breast cancer. Clin. Cancer Res. (in the press).
Hemsen, A. et al. Comparative evaluation of urokinase-type plasminogen activator receptor expression in primary breast carcinomas and metastatic tumor cells. Int. J. Cancer 107, 903–909 (2003).
Braun, S., Hepp, F., Sommer, H. -L. & Pantel, K. Tumor-antigen heterogeneity of disseminated breast cancer cells: implications for immunotherapy of minimal residual disease. Int. J. Cancer 84, 1–5 (1999).
Gebauer, G. et al. Epithelial cells in bone marrow of breast cancer patients at time of primary surgery: clinical outcome during long-term follow-up. J. Clin. Oncol. 19, 3669–3674 (2001).
Gerber, B. et al. Simultaneous immunohistochemical detection of tumor cells in lymph nodes and bone marrow aspirates in breast cancer and its correlation with other prognostic factors. J. Clin. Oncol. 19, 960–971 (2001).
Diel, I. J. et al. Micrometastatic breast cancer cells in bone marrow at primary surgery: prognostic value in comparison with nodal status. J. Natl Cancer Inst. 88, 1652–1658 (1996).
Mansi, J. L. et al. Outcome of primary breast cancer patients with micrometastases: a long term follow up study. Lancet 354, 197–202 (1999).
Acknowledgements
We apologize to those authors whose work we could not cite directly due to space constraints. The authors' research summarized here is mainly supported by the Deutsche Forschungsgemeinschaft (DFG), the Deutsche Krebshilfe, the Roggenbuck-Foundation, the Dutch Cancer Society, the Fanconi Anemia Research Fund and the Netherlands Organization for Scientific Research (NWO). The authors are recipients of a bi-national grant of the DFG and NWO.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Klaus Pantel is the co-founder of Micromet AG (Munich, Germany).
Related links
Glossary
- TUMOUR STAGE
-
Tumours are evaluated by a pathologist to determine their size and local extension into the surrounding tissue (pT-stage), along with metastases in the surgically removed regional lymph nodes (pN-stage). In addition, patients are screened for the presence of distant metastases (M-stage) by clinical means and radiological methods.
- EXPRESSION SIGNATURE
-
Levels of expression of a given set of genes in a specific tissue, usually assessed by comparing the tissue of interest to a control tissue by microarray analysis.
- DISSEMINATED TUMOUR CELLS
-
Single tumour cells or small cell clusters that can be detected in regional lymph nodes, peripheral blood or organs remote from the primary tumour. These cells are most commonly identified in the bone marrow by sensitive immunocytochemical and molecular techniques.
- MICRODISSECTION
-
Mechanical or laser-assisted dissection of a defined area or specific cells from a tissue section.
- CYTOKERATINS
-
Intermediate filaments of the cytoskeleton that are specifically expressed in epithelial cells.
- ILIAC CREST
-
The outer rim of the pelvic bone that is accessed for needle aspiration of bone marrow.
Rights and permissions
About this article
Cite this article
Pantel, K., Brakenhoff, R. Dissecting the metastatic cascade. Nat Rev Cancer 4, 448–456 (2004). https://doi.org/10.1038/nrc1370
Issue Date:
DOI: https://doi.org/10.1038/nrc1370