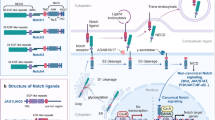
Key Points
-
Signalling between Notch receptors and ligands influences many differentiation processes and cell-fate decisions during embryonic and postnatal development.
-
Stem-cell maintenance, binary cell-fate decisions and induction of differentiation are three main functions of Notch signalling in self-renewing tissues.
-
Notch can function as an oncogene. Aberrant expression of the dominant active cytoplasmic domain of Notch receptors in haematopoietic cells because of chromosomal translocation or viral integrations causes T-cell leukaemias in mice and humans.
-
Notch needs to cooperate with oncoproteins that can override the G1–S checkpoint in order to cause cancer.
-
Notch receptors and ligands are re-expressed in certain human carcinomas, which is compatible with the ability of Notch to maintain stem cells or precursor cells in an undifferentiated state.
-
Recent data show that Notch1 can also function as a tumour suppressor in mouse skin by inducing Waf1 and repressing Shh and Wnt signalling.
-
Notch has two faces; one that promotes and the other that suppresses tumorigenesis. Which of the two faces is shown is dependent on the cellular context and the crosstalk with other signal-transduction pathways.
Abstract
Notch signalling participates in the development of multicellular organisms by maintaining the self-renewal potential of some tissues and inducing the differentiation of others. Involvement of Notch in cancer was first highlighted in human T-cell leukaemia, fuelling the notion that aberrant Notch signalling promotes tumorigenesis. However, there is mounting evidence that Notch signalling is not exclusively oncogenic. It can instead function as a tumour suppressor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
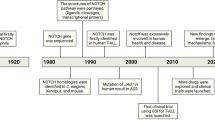
Morgan, T. H. The theory of the gene. Am. Nat. 51, 513–544 (1917).
Wharton, K. A. et al. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell 43, 567–581 (1985).
Kidd, S., Kelley, M. R. & Young, M. W. Sequence of the notch locus of Drosophila melanogaster: relationship of the encoded protein to mammalian clotting and growth factors. Mol. Cell Biol. 6, 3094–3108 (1986). References 2 and 3 are classic papers that describe the cloning of the Drosophila Notch gene.
Blaumueller, C. M. et al. Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell 90, 281–291 (1997).
Logeat, F. et al. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc. Natl Acad. Sci. USA 95, 8108–8112 (1998).
del Amo, F. F. et al. Cloning, analysis, and chromosomal localization of Notch-1, a mouse homolog of Drosophila Notch. Genomics 15, 259–264 (1993).
Weinmaster, G., Roberts, V. J. & Lemke, G. Notch2: a second mammalian Notch gene. Development 116, 931–941 (1992).
Lardelli, M. & Lendahl, U. Motch A and motch B: two mouse Notch homologues coexpressed in a wide variety of tissues. Exp. Cell Res. 204, 364–372 (1993).
Lardelli, M., Dahlstrand, J. & Lendahl, U. The novel Notch homologue mouse Notch 3 lacks specific epidermal growth factor-repeats and is expressed in proliferating neuroepithelium. Mech. Dev. 46, 123–136 (1994).
Uyttendaele, H. et al. Notch4/int-3, a mammary proto-oncogene, is an endothelial cell-specific mammalian Notch gene. Development 122, 2251–2259 (1996).
Bettenhausen, B. et al. Transient and restricted expression during mouse embryogenesis of Dll1, a murine gene closely related to Drosophila Delta. Development 121, 2407–2418 (1995).
Dunwoodie, S. L. et al. Mouse Dll3: a novel divergent Delta gene which may complement the function of other Delta homologues during early pattern formation in the mouse embryo. Development 124, 3065–3076 (1997).
Shutter, J. R. et al. Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev. 14, 1313–1318 (2000).
Lindsell, C. E. et al. Jagged: a mammalian ligand that activates Notch1. Cell 80, 909–917 (1995).
Shawber, C. et al. Jagged2: a serrate-like gene expressed during rat embryogenesis. Dev. Biol. 180, 370–376 (1996).
Kao, H. Y. et al. A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Genes Dev. 12, 2269–2277 (1998).
Hsieh, J. J. et al. CIR, a corepressor linking the DNA binding factor CBF1 to the histone deacetylase complex. Proc. Natl Acad. Sci. USA 96, 23–28 (1999).
Morel, V. et al. Transcriptional repression by suppressor of hairless involves the binding of a hairless-dCtBP complex in Drosophila. Curr. Biol. 11, 789–792 (2001).
Zhou, S. et al. SKIP, a CBF1-associated protein, interacts with the ankyrin repeat domain of NotchIC to facilitate NotchIC function. Mol. Cell Biol. 20, 2400–2410 (2000).
Kurooka, H. & Honjo, T. Functional interaction between the mouse notch1 intracellular region and histone acetyltransferases PCAF and GCN5. J. Biol. Chem. 275, 17211–11720 (2000).
Oswald, F. et al. p300 acts as a transcriptional coactivator for mammalian Notch-1. Mol. Cell Biol. 21, 7761–7774 (2001).
Fryer, C. J. et al. Mastermind mediates chromatin-specific transcription and turnover of the Notch enhancer complex. Genes Dev. 16, 1397–1411 (2002).
Bailey, A. M. & Posakony, J. W. Suppressor of hairless directly activates transcription of enhancer of split complex genes in response to Notch receptor activity. Genes Dev. 9, 2609–2622 (1995).
Davis, R. L. & Turner, D. L. Vertebrate hairy and Enhancer of split related proteins: transcriptional repressors regulating cellular differentiation and embryonic patterning. Oncogene 20, 8342–8357 (2001).
Rangarajan, A. et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 20, 3427–3436 (2001). This report shows that NOTCH1 induces numerous early differentiation markers and identifies the gene that encodes WAF1 as a direct transcriptional target.
Panin, V. M. & Irvine, K. D. Modulators of Notch signaling. Semin. Cell Dev. Biol. 9, 609–617 (1998).
Irvine, K. D. Fringe, Notch, and making developmental boundaries. Curr. Opin. Genet. Dev. 9, 434–441 (1999).
Varnum-Finney, B. et al. The Notch ligand, Jagged-1, influences the development of primitive hematopoietic precursor cells. Blood 91, 4084–4091 (1998).
Jaleco, A. C. et al. Differential effects of Notch ligands Delta-1 and Jagged-1 in human lymphoid differentiation. J. Exp. Med. 194, 991–1002 (2001).
Schmitt, T. M. & Zuniga-Pflucker, J. C. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity 17, 749–756 (2002).
Haltiwanger, R. S. & Stanley, P. Modulation of receptor signaling by glycosylation: fringe is an O-fucose-β1, 3-N-acetylglucosaminyltransferase. Biochim. Biophys. Acta 1573, 328–335 (2002).
Martinez Arias, A., Zecchini, V. & Brennan, K. CSL-independent Notch signalling: a checkpoint in cell fate decisions during development? Curr. Opin. Genet. Dev. 12, 524–533 (2002).
Brennan, K. & Gardner, P. Notching up another pathway. Bioessays 24, 405–410 (2002).
Lewis, J. Neurogenic genes and vertebrate neurogenesis. Curr. Opin. Neurobiol. 6, 3–10 (1996).
Lewis, J. Notch signalling and the control of cell fate choices in vertebrates. Semin. Cell Dev. Biol. 9, 583–589 (1998).
Chitnis, A. et al. Primary neurogenesis in Xenopus embryos regulated by a homologue of the Drosophila neurogenic gene Delta. Nature 375, 761–766 (1995).
Henrique, D. et al. Maintenance of neuroepithelial progenitor cells by Delta–Notch signalling in the embryonic chick retina. Curr. Biol. 7, 661–670 (1997).
Jones, P. et al. Stromal expression of Jagged 1 promotes colony formation by fetal hematopoietic progenitor cells. Blood 92, 1505–1511 (1998).
Kimble, J. & Simpson, P. The LIN-12/Notch signaling pathway and its regulation. Annu. Rev. Cell Dev. Biol. 13, 333–361 (1997).
Artavanis-Tsakonas, S., Matsuno, K. & Fortini, M. E. Notch signaling. Science 268, 225–232 (1995).
Morrison, S. J. et al. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell 101, 499–510 (2000).
Pear, W. S. & Radtke, F. Notch signaling in lymphopoiesis. Semin. Immunol. 15, 69–79 (2003).
Lowell, S. et al. Stimulation of human epidermal differentiation by delta–notch signalling at the boundaries of stem-cell clusters. Curr. Biol. 10, 491–500 (2000).
Reynolds, T. C., Smith, S. D. & Sklar, J. Analysis of DNA surrounding the breakpoints of chromosomal translocations involving the beta T cell receptor gene in human lymphoblastic neoplasms. Cell 50, 107–117 (1987).
Ellisen, L. W. et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 66, 649–661 (1991). This paper identified the first human homologue of the Drosophila Notch gene and its truncated form in the chromosomal translocation t(7;9)(q34;q34. 3) from a human T-lymphoblastic leukaemia. Furthermore, it is the first paper to indicate that aberrant expression of the cytoplasmic part of the human NOTCH1 gene causes T-cell neoplasm.
Pear, W. S. et al. Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. J. Exp. Med. 183, 2283–2291 (1996). This is the first report showing the oncogenic potential of the truncated human NOTCH1 gene in the haematopoietic compartment using a mouse model.
Pui, J. C. et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity 11, 299–308 (1999).
Radtke, F. et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 10, 547–558 (1999).
Izon, D. J. et al. Deltex1 redirects lymphoid progenitors to the B cell lineage by antagonizing Notch1. Immunity 16, 231–243 (2002).
Aster, J. C. et al. Essential roles for ankyrin repeat and transactivation domains in induction of T-cell leukemia by notch1. Mol. Cell Biol. 20, 7505–7015 (2000).
Girard, L. et al. Frequent provirus insertional mutagenesis of Notch1 in thymomas of MMTVD/myc transgenic mice suggests a collaboration of c-myc and Notch1 for oncogenesis. Genes Dev. 10, 1930–1944 (1996).
Feldman, B. J. Hampton, T. & Cleary, M. L. A carboxy-terminal deletion mutant of Notch1 accelerates lymphoid oncogenesis in E2A-PBX1 transgenic mice. Blood 96, 1906–1913 (2000).
Beverly, L. J. & Capobianco, A. J. Perturbation of Ikaros isoform selection by MLV integration is a cooperative event in Notch(IC)-induced T cell leukemogenesis. Cancer Cell 3, 551–564 (2003).
Allman, D. et al. Separation of Notch1 promoted lineage commitment and expansion/transformation in developing T cells. J. Exp. Med. 194, 99–106 (2001). This report shows that aberrant Notch signalling in haematopoietic progenitors has to cooperate with a T-cell-specific signal (pre-TCR mediated signal) in order to cause T-cell neoplasms.
Robey, E. et al. An activated form of Notch influences the choice between CD4 and CD8 T cell lineages. Cell 87, 483–492 (1996).
Bellavia, D. et al. Constitutive activation of NF-κB and T-cell leukemia/lymphoma in Notch3 transgenic mice. EMBO J. 19, 3337–3348 (2000).
Deftos, M. L. et al. Notch1 signaling promotes the maturation of CD4 and CD8 SP thymocytes. Immunity 13, 73–84 (2000).
Rohn, J. L. et al. Transduction of Notch2 in feline leukemia virus-induced thymic lymphoma. J. Virol. 70, 8071–8080 (1996).
Yan, X. Q. et al. A novel Notch ligand, Dll4, induces T-cell leukemia/lymphoma when overexpressed in mice by retroviral-mediated gene transfer. Blood 98, 3793–3799 (2001).
Dorsch, M. et al. Ectopic expression of Delta4 impairs hematopoietic development and leads to lymphoproliferative disease. Blood 100, 2046–2055 (2002).
Weng, A. P. et al. Growth suppression of pre-T acute lymphoblastic leukemia cells by inhibition of notch signaling. Mol. Cell Biol. 23, 655–664 (2003).
Gallahan, D. & Callahan, R. Mammary tumorigenesis in feral mice: identification of a new int locus in mouse mammary tumor virus (Czech II)-induced mammary tumors. J. Virol. 61, 66–74 (1987).
Gallahan, D. et al. Expression of a truncated Int3 gene in developing secretory mammary epithelium specifically retards lobular differentiation resulting in tumorigenesis. Cancer Res. 56, 1775–1785 (1996).
Jhappan, C. et al. Expression of an activated Notch-related int-3 transgene interferes with cell differentiation and induces neoplastic transformation in mammary and salivary glands. Genes Dev. 6, 345–355 (1992). Shows that aberrant expression of the int3 locus, which was later identifed as the NOTCH4 gene (see also reference 9), can cause epithelial tumours.
Dievart, A., Beaulieu, N. & Jolicoeur, P. Involvement of Notch1 in the development of mouse mammary tumors. Oncogene 18, 5973–5981 (1999).
Weijzen, S. et al. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nature Med. 8, 979–986 (2002). Shows that the presence of NOTCH1 is not a consequence of cancer, but is instead required for the manifestation of cancer properties.
Zagouras, P. et al. Alterations in Notch signaling in neoplastic lesions of the human cervix. Proc. Natl Acad. Sci. USA 92, 6414–6418 (1995). This is the first report that alluded to the involvement of Notch in carcinomas, specifically cervical cancers.
Milner, L. A. et al. Inhibition of granulocytic differentiation by mNotch1. Proc. Natl Acad. Sci. USA 93, 13014–13019 (1996).
Milner, L. A. & Bigas, A. Notch as a mediator of cell fate determination in hematopoiesis: evidence and speculation. Blood 93, 2431–2448 (1999).
Ronchini, C. & Capobianco, A. J. Notch(ic)-ER chimeras display hormone-dependent transformation, nuclear accumulation, phosphorylation and CBF1 activation. Oncogene 19, 3914–3924 (2000).
Dumont, E. et al. Neoplastic transformation by Notch is independent of transcriptional activation by RBP-J signalling. Oncogene 19, 556–561 (2000).
Jeffries, S. & Capobianco, A. J. Neoplastic transformation by Notch requires nuclear localization. Mol. Cell Biol. 20, 3928–3941 (2000).
Imatani, A. & Callahan, R. Identification of a novel NOTCH-4/INT-3 RNA species encoding an activated gene product in certain human tumor cell lines. Oncogene 19, 223–231 (2000).
Capobianco, A. J. et al. Neoplastic transformation by truncated alleles of human NOTCH1/TAN1 and NOTCH2. Mol. Cell Biol. 17, 6265–6273 (1997).
Rangarajan, A. et al. Activated Notch1 signaling cooperates with papillomavirus oncogenes in transformation and generates resistance to apoptosis on matrix withdrawal through PKB/Akt. Virology 286, 23–30 (2001).
Fitzgerald, K., Harrington, A. & Leder, P. Ras pathway signals are required for notch-mediated oncogenesis. Oncogene 19, 4191–4198 (2000).
Bocchetta, M. et al. Notch-1 induction, a novel activity of SV40 required for growth of SV40-transformed human mesothelial cells. Oncogene 22, 81–89 (2003).
Chen, Y., Fischer, W. H. & Gill, G. N. Regulation of the ERBB-2 promoter by RBPJkappa and NOTCH. J. Biol. Chem. 272, 14110–14114 (1997).
Oswald, F. et al. NF-κB2 is a putative target gene of activated Notch-1 via RBP-Jκ. Mol. Cell Biol. 18, 2077–2088 (1998).
Frisch, S. M. & Francis, H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 124, 619–626 (1994).
Khwaja, A. et al. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 16, 2783–2793 (1997).
Singh, B. et al. p53 regulates cell survival by inhibiting PIK3CA in squamous cell carcinomas. Genes Dev. 16, 984–993 (2002).
Stern, D. F. ErbBs in mammary development. Exp. Cell Res. 284, 89–98 (2003).
Lohrisch, C. & Piccart, M. An overview of HER2. Semin. Oncol. 28, 3–11 (2001).
Yarden, Y. Biology of HER2 and its importance in breast cancer. Oncology 61 (Suppl 2), 1–13 (2001).
Lin, A. & Karin, M. NF-κB in cancer: a marked target. Semin. Cancer Biol. 13, 107–114 (2003).
Klein, G., Powers, A. & Croce, C. Association of SV40 with human tumors. Oncogene 21, 1141–1149 (2002).
Shivapurkar, N. et al. Presence of simian virus 40 sequences in malignant mesotheliomas and mesothelial cell proliferations. J. Cell Biochem. 76, 181–188 (1999).
Garcea, R. L. & Imperiale, M. J. Simian virus 40 infection of humans. J. Virol. 77, 5039–5045 (2003).
Nickoloff, B. J. et al. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-κB and PPARγ. Cell Death Differ. 9, 842–855 (2002).
Talora, C. et al. Specific down-modulation of Notch1 signaling in cervical cancer cells is required for sustained HPV-E6/E7 expression and late steps of malignant transformation. Genes Dev. 16, 2252–2263 (2002).
Chu, J. et al. Repression of activator protein-1-mediated transcriptional activation by the Notch-1 intracellular domain. J. Biol. Chem. 277, 7587–7597 (2002).
Nicolas, M. et al. Notch1 functions as a tumor suppressor in mouse skin. Nature Genet. 33, 416–421 (2003). Shows that inactivation of Notch1 in the skin results in the development of basal-cell-carcinoma-like tumours due to downregulation of Waf1 and repression of Gli2-mediated Sonic hedgehog signalling and β-catenin-mediated Wnt signalling.
Topley, G. I. et al. p21(WAF1/Cip1) functions as a suppressor of malignant skin tumor formation and a determinant of keratinocyte stem-cell potential. Proc. Natl Acad. Sci. USA 96, 9089–9094 (1999).
Weinberg, W. C. et al. Genetic deletion of p21WAF1 enhances papilloma formation but not malignant conversion in experimental mouse skin carcinogenesis. Cancer Res. 59, 2050–2054 (1999).
Philipp, J. et al. Tumor suppression by p27Kip1 and p21Cip1 during chemically induced skin carcinogenesis. Oncogene 18, 4689–4698 (1999).
Thelu, J., Rossio, P. & Favier, B. Notch signalling is linked to epidermal cell differentiation level in basal cell carcinoma, psoriasis and wound healing. BMC Dermatol. 2, 7 (2002).
Polakis, P. Wnt signaling and cancer. Genes Dev. 14, 1837–1851 (2000).
Lo Muzio, L. et al. WNT-1 expression in basal cell carcinoma of head and neck. An immunohistochemical and confocal study with regard to the intracellular distribution of β-catenin. Anticancer Res. 22, 565–576 (2002).
Yamazaki, F. et al. Immunohistochemical detection for nuclear β-catenin in sporadic basal cell carcinoma. Br. J. Dermatol. 145, 771–777 (2001).
Boonchai, W. et al. Expression of β-catenin, a key mediator of the WNT signaling pathway, in basal cell carcinoma. Arch. Dermatol. 136, 937–938 (2000).
Axelrod, J. D. et al. Interaction between Wingless and Notch signaling pathways mediated by dishevelled. Science 271, 1826–1832 (1996).
Borges, M. et al. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature 386, 852–855 (1997).
Ito, T. et al. Basic helix–loop–helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development 127, 3913–3921 (2000).
de la Pompa, J. L. et al. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development 124, 1139–1148 (1997).
Ishibashi, M. et al. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix–loop–helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 9, 3136–3148 (1995).
Sriuranpong, V. et al. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res. 61, 3200–3205 (2001).
Sriuranpong, V. et al. Notch signaling induces rapid degradation of achaete-scute homolog 1. Mol. Cell Biol. 22, 3129–3139 (2002).
Field, J. K. & Spandidos, D. A. The role of ras and myc oncogenes in human solid tumours and their relevance in diagnosis and prognosis. Anticancer Res. 10, 1–22 (1990).
Bos, J. L. The ras gene family and human carcinogenesis. Mutat Res. 195, 255–271 (1988).
Land, H., Parada, L. F. & Weinberg, R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature 304, 596–602 (1983).
Serrano, M. et al. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
Evan, G. I. et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell 69, 119–128 (1992). The first report to show that an oncogene can induce apoptosis. This led to many subsequent reports that showed growth-suppressing properties of oncogenes.
Walboomers, J. M. et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189, 12–19 (1999).
Scheffner, M. et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63, 1129–1136 (1990).
Dyson, N. et al. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243, 934–937 (1989).
Munger, K. et al. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene 20, 7888–7898 (2001).
Klingelhutz, A. J., Foster, S. A. & McDougall, J. K. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 380, 79–82 (1996).
Daniel, B. et al. The link between integration and expression of human papillomavirus type 16 genomes and cellular changes in the evolution of cervical intraepithelial neoplastic lesions. J. Gen. Virol. 78, 1095–1101 (1997).
Helt, A. M., Funk, J. O. & Galloway, D. A. Inactivation of both the retinoblastoma tumor suppressor and p21 by the human papillomavirus type 16 E7 oncoprotein is necessary to inhibit cell cycle arrest in human epithelial cells. J. Virol. 76, 10559–10568 (2002).
Westbrook, T. F. et al. E7 abolishes raf-induced arrest via mislocalization of p21(Cip1). Mol. Cell. Biol. 22, 7041–7052 (2002).
Bedell, M. A., Jones, K. H. & Laimins, L. A. The E6-E7 region of human papillomavirus type 18 is sufficient for transformation of NIH 3T3 and rat-1 cells. J. Virol. 61, 3635–3640 (1987).
Crook, T. et al. Continued expression of HPV-16 E7 protein is required for maintenance of the transformed phenotype of cells co-transformed by HPV-16 plus EJ-ras. EMBO J. 8, 513–519 (1989).
Ma, Y. Y. et al. PIK3CA as an oncogene in cervical cancer. Oncogene 19, 2739–2744 (2000).
Yang, A. et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell 2, 305–316 (1998).
Hibi, K. et al. AIS is an oncogene amplified in squamous cell carcinoma. Proc. Natl Acad. Sci. USA 97, 5462–5467 (2000).
Heselmeyer, K. et al. Gain of chromosome 3q defines the transition from severe dysplasia to invasive carcinoma of the uterine cervix. Proc. Natl Acad. Sci. USA 93, 479–484 (1996).
Rooney, P. H. et al. Comparative genomic hybridization and chromosomal instability in solid tumours. Br. J. Cancer 80, 862–873 (1999).
Wang, T. Y. et al. Histologic and immunophenotypic classification of cervical carcinomas by expression of the p53 homologue p63: a study of 250 cases. Hum. Pathol. 32, 479–486 (2001).
Quade, B. J. et al. Expression of the p53 homologue p63 in early cervical neoplasia. Gynecol. Oncol. 80, 24–29 (2001).
Sharma, M., Chuang, W. W. & Sun, Z. Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3β inhibition and nuclear β-catenin accumulation. J. Biol. Chem. 277, 30935–30941 (2002).
Patturajan, M. et al. δNp63 induces β-catenin nuclear accumulation and signaling. Cancer Cell 1, 369–379 (2002).
Price, M. A. & Kalderon, D. Proteolysis of the hedgehog signaling effector Cubitus interruptus requires phosphorylation by glycogen synthase kinase 3 and casein kinase 1. Cell 108, 823–835 (2002).
Kalderon, D. Similarities between the Hedgehog and Wnt signaling pathways. Trends Cell Biol. 12, 523–531 (2002).
Jia, J. et al. Shaggy/GSK3 antagonizes Hedgehog signalling by regulating Cubitus interruptus. Nature 416, 548–552 (2002).
Hahn, W. C. et al. Creation of human tumour cells with defined genetic elements. Nature 400, 464–468 (1999).
Lathion, S., Schaper, J., Beard, P. & Raj, K. Notch1 can contribute to viral-induced transformation of primary human keratinocytes. Cancer Res. (in the press).
Ruiz i Altaba, A., Sanchez, P. & Dahmane, N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nature Rev. Cancer 2, 361–372 (2002).
Acknowledgements
We apologize to colleagues whose work was not referenced because of space limitations. Research in the Radtke lab is supported by grants from the Swiss Cancer League, the National Science Foundation, the Leenaards Foundation and the European Molecular Biology Organization Young Investigator Programme. Research in the Raj lab is supported by the National Centre of Competence in Research programme of the Swiss National Science Foundation. We thank J. Hornfeld and M. Migliaccio for careful reading of the manuscript and comments. We are also grateful to the anonymous referees, whose constructive suggestions helped improve this review.
Author information
Authors and Affiliations
Glossary
- HAPLOINSUFFICIENCY
-
A situation in which a loss-of-function phenotype is produced by mutation of one allele of a gene in a diploid cell, even though the other allele is wild type.
- FUCOSE RESIDUES
-
Sugar residues that are attached to certain EGF repeats of Notch receptors. In the presence of the corresponding saccharides, Fringe proteins can elongate these sugar chains.
- BINARY CELL-FATE DECISION
-
The situation in which a precursor cell has to choose between two different cell fates.
- MYELOID ORIGIN
-
Cells of myeloid origin include macrophages, granulocytes, megakaryocytes, erythroblasts and myeloid-dentritic cells, whereas cells of lymphoid origin include T cells, B cells, natural-killer cells and lymphoid dendritic cells.
- ERBB2
-
A receptor tyrosine kinase that is overexpressed and activated in many tumours, especially in breast cancers.
- ANOIKIS
-
Cell death induced as a result of the absence of matrix attachment.
- SUPRABASAL LAYERS
-
The layers of the skin comprising the spinous and the granular layers.
- SENESCENCE
-
The irreversible loss of the proliferation potential, mainly caused by shortening and dysfunction of telomeres. However, it can also be caused by suboptimal culture condition, termed culture shock.
- p63
-
p63 is a homologue of the tumour suppressor p53 and the related protein p73. The TP63 gene encodes several isotypes with divergent abilities to transactivate p53 reporter genes and to induce apoptosis. The predominant p63 isotypes in many epithelial tissues lack the acidic amino terminus (so are known as ΔNp63), which corresponds to the transactivation domain of p53, and therefore lack transactivation function.
Rights and permissions
About this article
Cite this article
Radtke, F., Raj, K. The role of Notch in tumorigenesis: oncogene or tumour suppressor?. Nat Rev Cancer 3, 756–767 (2003). https://doi.org/10.1038/nrc1186
Issue Date:
DOI: https://doi.org/10.1038/nrc1186
This article is cited by
-
Bioinformatic analysis of m6A “reader” YTH family in pan-cancer as a clinical prognosis biomarker
Scientific Reports (2023)
-
Angiogenic signaling pathways and anti-angiogenic therapy for cancer
Signal Transduction and Targeted Therapy (2023)
-
LINC00460 promotes neuroblastoma tumorigenesis and cisplatin resistance by targeting miR-149-5p/DLL1 axis and activating Notch pathway in vitro and in vivo
Drug Delivery and Translational Research (2023)
-
Lentinan enhances the antitumor effects of Delta-like 1 via neutrophils
BMC Cancer (2022)
-
NDR1 increases NOTCH1 signaling activity by impairing Fbw7 mediated NICD degradation to enhance breast cancer stem cell properties
Molecular Medicine (2022)