Key Points
-
Liver cancer is the third most deadly cancer worldwide and is ranked fifth in terms of the number of cases.
-
Carcinogenic toxins produced by moulds (aflatoxins) interact synergistically with infection with hepatitis B virus (HBV) to amplify the risk of hepatocellular carcinoma (HCC) in humans — particularly in the developing world, where most of the disease burden lies.
-
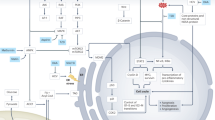
Molecular-progression pathways have been difficult to elucidate for HCC. However, several genetic and epigenetic changes in targets that affect the fate and actions of the viral and chemical aetiological agents offer promise as aids for early diagnosis.
-
Primary prevention of HCC, in the form of universal vaccination programmes against HBV in newborns, is proving effective, but it remains beyond the reach of many at-risk populations.
-
Secondary prevention of HCC, in the form of chemoprevention directed at many targets, offers potential benefits. Early-stage interventions designed to intercept and enhance the detoxification of aflatoxins, and later-stage interventions to inhibit or reverse cirrhosis or block the occurrence of second primary HCCs, are important opportunities for clinical-trial development.
Abstract
Unlike many other types of human cancer, the aetiology of liver cancer is well understood. Infection with hepatitis viruses, coupled with dietary exposure to the fungal toxin aflatoxin, increases the risk of the disease. Although primary prevention, based on vaccination and avoiding exposure to these agents, is an appealing option, such strategies will require considerable investment of time and resources to be successful. In the developing world — where the burden of liver cancer is highest — immediate, practical and economical approaches are essential. So, targeted chemoprevention might be most appropriate for the present generation of individuals at risk.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Parkin, D. M., Pisani, P. & Ferlay, J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int. J. Cancer 80, 827–841 (1999).
Li, L. & Rao, K. (eds) Cancer Incidence and Mortality in Cities and Counties of P. R. China 1988–1992 (China Medical Science and Technology Press, 2001).
Jemel, A. et al. Cancer statistics 2003. CA Cancer J. Clin. 53, 5–26 (2003).
World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition and the Prevention of Cancer: a Global Perspective (American Institute for Cancer Research, Washington DC, 1997).
Chen, J. -G. et al. Population-based cancer survival in Qidong, People's Republic of China. IARC Sci. Publ. 145, 27–35 (1998).
Merican, I. et al. Chronic hepatitis B virus infection in Asian countries. J. Gastroenterol. Hepatol. 15, 1356–1361 (2000).
Ogunbiyi, J. O. Hepatocellular carcinoma in the developing world. Semin. Oncol. 28, 179–187 (2001).
Etzel, R. A. Mycotoxins. JAMA 287, 425–427 (2002).
Aflatoxins. IARC Monograph on the Evaluation of Carcinogenic Risks to Humans 82, 171–300 (2002).
Hahn, W. C. & Weinberg, R. A. Modeling the molecular circuitry of cancer. Nature Rev. Cancer 2, 331–341 (2002).
Vogelstein, B. & Kinzler, K. W. (eds) The Genetic Basis of Human Cancer 2nd edn (McGraw–Hill, New York, 2002).
Herman, J. G. Hypermethylation pathways to colorectal cancer. Implications for prevention and detection. Gastroenterol. Clin. North Am. 31, 945–958 (2002).
Thorgeirsson, A. & Grisham, J. W. Molecular pathogenesis of human hepatocellular carcinoma. Nature Genet. 31, 339–346 (2002).
Seeger, C. & Mason, W. S. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 64, 51–68 (2000).
Hoofnagle, J. H. & Schafer, D. F. Serologic markers of hepatitis B virus infection. Semin. Liver Dis. 6, 1–10 (1986).
Weiland, O. & Schvarcz, R. Hepatitis C: virology, epidemiology, clinical course, and treatment. Scand. J. Gastroenterol. 27, 337–342 (1992).
Plaa, G. L. & Hewitt, W. R. in Principles and Methods of Toxicology 2nd edn (ed. Hayes, A. W.) 599–628 (Raven Press, New York, 1989).
Colombo, M. Hepatitis C virus and hepatocellular carcinoma. Baillière's Clin. Gastroenterol. 13, 519–528 (1999).
El-Serag, H. B. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology 36, S75–S83 (2002).
Zein, C. O. & Zein, N. N. Advances in therapy for hepatitis C infection. Microbes Infect. 4, 1237–1246 (2002).
Essigmann, J. M. et al. Structural identification of the major DNA adduct formed by aflatoxin B1 in vitro. Proc. Natl Acad. Sci. USA 74, 1870–1874 (1977).
Sabbioni, G., Skipper, P. L., Büchi, G. & Tannenbaum, S. R. Isolation and characterization of the major serum albumin adduct formed by aflatoxin B1 in vivo in rats. Carcinogenesis 8, 819–824 (1987).
Bennett, R. A., Essigmann, J. M. & Wogan, G. N. Excretion of an aflatoxin–guanine adduct in the urine of aflatoxin B1-treated rats. Cancer Res. 41, 650–654 (1981).
Ross, R. K. et al. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet 339, 943–946 (1992). This study was the first to show the synergistic interaction between aflatoxin exposure and infection with hepatitis B virus (HBV) in the aetiology of hepatocellular carcinoma (HCC).
Qian, G. -S. et al. A follow-up study of urinary markers of aflatoxin exposure and liver cancer in Shanghai, People's Republic of China. Cancer Epidemiol. Biomarkers Prev. 3, 3–10 (1994).
Sun, Z. -T. et al. Increased risk of hepatocellular carcinomain male hepatitis B surface antigen carriers with chronic hepatitis who have detectable urinary aflatoxin metabolite M1. . Hepatology 30, 379–383 (1999).
Wang, L. -Y. et al. Aflatoxin exposure and the risk of hepatocellular carcinoma in Taiwan. Int. J. Cancer 67, 620–625 (1996).
Yu, M. W. et al. Effect of aflatoxin metabolism and DNA adduct formation on hepatocellular carcinoma among chronic hepatitis B carriers in Taiwan. J. Hepatol. 27, 320–330 (1997).
Okuda, K. Clinical and public health challenges of cancer. Natural history of hepatocellular carcinoma including fibrolamellar and hepato-cholangiocarcinoma variants. J. Gastroenterol. Hepatol. 17, 401–405 (2002).
Johnson, P. J. The role of serum α-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin. Liver Dis. 5, 145–159 (2001).
Chen, J. -G. et al. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J. Med. Screening (in the press).
Kim, J. W. & Wang, X. W. Gene expression profiling of preneoplastic liver disease and liver cancer: a new era for improved early detection and treatment of these deadly diseases? Carcinogenesis 24, 363–369 (2003).
Nawroz, H. et al. Microsatellite alterations in serum DNA of head and neck cancer patients. Nature Med. 2, 1035–1037 (1996).
Yamada, T. et al. Detection of K-ras gene mutations in plasma DNA of patients with pancreatic adenocarcinoma: correlation with clinicopathological features. Clin. Cancer Res. 4, 1527–1532 (1998).
Esteller, M. et al. Detection of aberrant promoter hypermethylation of tumor suppressor genes in serum DNA from non-small cell lung cancer patients. Cancer Res. 59, 67–70 (1999).
Wong, I. H. et al. Detection of aberrant p16 methylation in the plasma and serum of liver cancer patients. Cancer Res. 59, 71–73 (1999).
Anker, P., Mulcahy, H., Chen, X. Q. & Stroun, M. Detection of circulating tumor DNA in the blood (plasma/serum) of cancer patients. Cancer Metastasis Rev. 18, 65–73 (1999).
Wong, I. H., Lo, Y. M., Yeo, W., Lau, W. Y. & Johnson, P. J. Frequent p15 promoter methylation in tumor and peripheral blood from hepatocellular carcinoma patients. Clin. Cancer Res. 6, 3516–3521 (2000).
Zhong, S. et al. Silencing of GSTP1 gene by CpG island DNA hypermethylation in HBV-associated hepatocellular carcinomas. Clin. Cancer Res. 8, 1087–1092 (2002). Epigenetic silencing of a gene encoding a carcinogen-detoxification enzyme, GSTP1, is associated with the pathogenesis of HCC.
Hsia, C. C., Yowen, H. & Tabor, E. Hot-spot mutations in hepatitis B virus X gene in hepatocellular carcinoma. Lancet 348, 625–626 (1996).
Ming, L. et al. Dominant role of hepatitis B virus and cofactor role of aflatoxin in hepatocarcinogenesis in Qidong, China. Hepatology 36, 1214–1220 (2002). A comprehensive assessment of the separate and combined effects of HBV, hepatitis C virus (HCV) and aflatoxin in causing HCC in Qidong, China.
Kuang, S. -Y. et al. Detection of specific mutations in the hepatitis B virus X-gene associated with codon 249 mutations in p53 in hepatocellular carcinoma patients. Proc. Am. Assoc. Cancer Res. 44, 593 (2003).
Wang, X. W. et al. Molecular pathogenesis of human hepatocellular carcinoma. Toxicology 181–182, 43–47 (2002).
Wild, C. P. & Turner, P. C. The toxicology of aflatoxins as a basis for public health decisions. Mutagenesis 17, 471–481 (2002).
Staib, F. et al. TP53 and liver carcinogenesis. Hum. Mutat. 21, 201–216 (2003).
Kirk, G. D. et al. Ser-249 p53 mutations in plasma DNA of patients with hepatocellular carcinoma from The Gambia. J. Natl Cancer Inst. 92, 148–153 (2000). The first demonstration that mutations of TP53 can be detected in plasma samples of patients with HCC.
Jackson, P. E. et al. Specific p53 mutation detected in plasma and tumors of hepatocellular carcinoma patients by electrospray ionization mass spectrometry. Cancer Res. 61, 33–35 (2001).
Jackson, P. E. et al. Prospective detection of codon 249 mutations in p53 in plasma of hepatocellular carcinoma patients. Carcinogenesis (in the press).
Wild, C. P. & Turner, P. C. in Biomarkers in Cancer Chemoprevention (eds Miller, A. B. et al.) 154, 215–222 (International Agency for Research on Cancer by Oxford University Press, UK, 2001).
Sun, T. -T. et al. A pilot study on universal immunization of newborn infants in an area of hepatitis B virus and primary hepatocellular carcinoma prevalence with a low dose of hepatitis B vaccine. J. Cell. Physiol. 4, 83–90 (1986).
Chang, M. -H. et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N. Engl. J. Med. 336, 1855–1859 (1997). The initial report describing the efficacy of HBV vaccination in reducing the incidence of HCC.
Lee, C. -L., Hsieh, K. -S. & Ko, Y. -C. Trends in the incidence of hepatocellular carcinoma in boys and girls in Taiwan after large-scale hepatitis B vaccination. Cancer Epidemiol. Biomarkers Prev. 12, 57–59 (2003).
Busby, W. F. Jr & Wogan, G. N. in Chemical Carcinogenesis 2nd edn, Vol. 2 (ed. Searle, C. E.) 945–1136 (ACS, Washington DC, 1984).
Turner, P. C. et al. The role of aflatoxins and hepatitis viruses in the etiopathogenesis of hepatocellular carcinoma: a basis for primary prevention in Guinea-Conakry, West Africa. J. Gastroenterol. Hepatol. 17, S441–S448 (2002). This study describes the development and use of biomarkers to assess the impact of reduction of aflatoxin exposure on the development of HCC in high-risk populations.
Sporn, M. B. & Suh, N. Chemoprevention: an essential approach to controlling cancer. Nature Rev. Cancer 2, 537–543 (2002).
Gupta, R. A. & DuBois, R. N. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nature Rev. Cancer 1, 11–21 (2001).
Dashwood, R. et al. Chemopreventive properties of chlorophylls towards aflatoxin B1. A review of the antimutagenicity and anticarcinogenicity data in rainbow trout. Mutat. Res. 399, 245–253 (1998).
Kephart, J. C. Chlorophyll derivatives — their chemistry, commercial preparation and uses. Econ. Bot. 9, 3–38 (1955).
Young, R. W. & Bergei, J. S. Use of chlorophyllin in the care of geriatric patients. J. Am. Geriat. Soc. 24, 46–47 (1980).
Breinholt, V., Schimerlik, M., Dashwood, R. & Bailey, G. Mechanisms of chlorophyllin anticarcinogenesis against aflatoxin B1. Complex formation with the carcinogen. Chem. Res. Toxicol. 8, 506–514 (1995).
Egner, P. A. et al. Chlorophyllin intervention reduces aflatoxin–DNA adducts in individuals at high risk for liver cancer. Proc. Natl Acad. Sci. USA 98, 14601–14606 (2001). A pivotal clinical trial showing that an 'interceptor' molecule, chlorophyllin, can be used to reduce the bioavailability of aflatoxin in humans.
Bergquist, N. R. Schistosomiasis: from risk assessment to control. Trends Parasitol. 18, 309–314 (2002).
Wang, J. -S. et al. Protective alterations in phase 1 and 2 metabolism of aflatoxin B1 by oltipraz in residents of Qidong, People's Republic of China. J. Natl Cancer Inst. 91, 347–354 (1999). This study showed that oltipraz can modulate the effects of aflatoxin in humans through the induction of carcinogen-detoxification enzymes.
Kensler, T. W. et al. Oltipraz chemoprevention trial in Qidong, People's Republic of China: modulation of serum aflatoxin albumin adduct biomarkers. Cancer Epidemiol. Biomarkers Prev. 7, 127–134 (1998).
Talalay, P. & Talalay, P. The importance of using scientific principles in the development of medicinal agents from plants. Acad. Med. 76, 238–247 (2001).
Fahey, J. W., Zalcmann, A. T. & Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56, 5–51 (2001).
Zhang, Y., Talalay, P., Cho, C. G. & Posner, G. H. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc. Natl Acad. Sci. USA 89, 2399–2403 (1992).
Fahey, J. W., Zhang, Y. & Talalay, P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl Acad. Sci. USA 94, 10367–10372 (1997). The initial demonstration that high levels of the anti-carcinogen isothiocyanate sulphoraphane can be found in 3-day-old broccoli sprouts.
Shapiro, T. et al. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer Epidemiol. Biomarkers Prev. 10, 501–508 (2001).
Bogaards, J. J., Verhagen, H., Willems, M. I., van Poppel, G. & van Bladdern, P. J. Consumption of Brussels sprouts results in elevated α-class glutathione S-transferase levels in human blood plasma. Carcinogenesis 15, 1073–1075 (1994).
Lampe, J. W. et al. Modulation of human glutathione S-transferases by botanically defined vegetable diets. Cancer Epidemiol. Biomarkers Prev. 9, 787–793 (2000).
Primiano, T. et al. Intermittent dosing with oltipraz: relationship between chemoprevention of aflatoxin-induced tumorigenesis and induction of glutathione S-transferases. Cancer Res. 55, 4319–4324 (1995).
Nakachi, K., Matsuyama, S., Miyake, S., Suganuma, M. & Imai, K. Preventive effects of drinking green tea on cancer and cardiovascular disease: epidemiological evidence for multiple targeting prevention. BioFactors 13, 49–54 (2000).
Fujiki, H., Suganuma, M., Imai, K. & Nakachi, K. Green tea: cancer preventive beverage and/or drug. Cancer Lett. 188, 9–13 (2002).
Wang, J. -S. et al. Chemoprevention trial of green tea polyphenols in high-risk population of liver cancer. Proc. Am. Assoc. Cancer Res. 44, 1101 (2003).
Klaunig, J. E. et al. The effect of tea consumption on oxidative stress in smokers and nonsmokers. Proc. Soc. Exp. Biol. Med. 220, 249–254 (1999).
Kang, K. W. et al. Oltipraz regenerates cirrhotic liver through CCAAT/enhancer binding protein-mediated stellate cell inactivation. FASEB J. 16, 1988–1990 (2002).
Muto, Y. et al. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma: hepatoma prevention study group. N. Engl. J. Med. 334, 1561–1567 (1996). This study describes a clinical chemoprevention trial of polyprenoic acid in a Japanese cohort. HCV infection was the main cause of HCC in more than 70% of patients in the study population. Minimal side effects were reported for 600 mg of polyprenoic acid per day — a treatment that significantly prevented second primary tumours.
Okuno, M., Kojima, S. & Moriwaki, H. Chemoprevention of hepatocellular carcinoma: concept, progress and perspectives. J. Gastroenterol. Hepatol. 16, 1329–1335 (2001).
Lippman, S. M. & Hong, W. K. Cancer prevention by delay. Clin. Cancer Res. 8, 305–313 (2002).
Kensler, T. W. et al. Predictive value of molecular dosimetry: individual versus group effects of oltipraz on aflatoxin–albumin adducts and risk of hepatocellular carcinoma. Cancer Epidemiol. Biomarkers Prev. 6, 603–610 (1997).
Loeb, L. A., Loeb, K. A. & Anderson, J. P. Multiple mutations and cancer. Proc. Natl Acad. Sci. USA 100, 776–781 (2003).
Sun, Z. & Zhu, Y. -R. Multifactorial etiology and multifaceted prevention strategy of hepatocellular carcinoma. Cancer Detect. Prev. 14, 285–289 (1989).
Kensler, T. W., Groopman, J. D., Sutter, T. R., Curphey, T. J. & Roebuck, B. D. Development of cancer chemopreventive agents: oltipraz as a paradigm. Chem. Res. Toxicol. 12, 113–126 (1999).
Kwak, M. -K. et al. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1–Nrf2 pathway. Identification of novel gene clusters for cell survival. J. Biol. Chem. 278, 8135–8145 (2003).
Itoh, K. et al. A Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 236, 313–322 (1997).
Itoh, K. et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 13, 76–86 (1999).
Dinkova-Kostova, A. T. et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl Acad. Sci. USA 99, 11908–11913 (2002).
Ramos-Gomez, M., Dolan, P. M., Itoh, K., Yamamoto, M. & Kensler, T. W. Interactive effects of nrf2 genotype and oltipraz on benzo[a]pyrene–DNA adducts and tumor yield in mice. Carcinogenesis 24, 461–467 (2003).
Ramos-Gomez, M. et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl Acad. Sci. USA 98, 3410–3415 (2001).
Fahey, J. W. et al. Sulforaphane inhibits extracellular, intracellular, and antiobiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl Acad. Sci. USA 99, 7610–7615 (2002).
Acknowledgements
Our work is supported in part by United States Public Health Services grants from the National Cancer Institute and the National Institute of Environmental Health Sciences, as well as by the Science & Technology Commission of Shanghai Municipality, Shanghai Municipal Health Bureau and Jiangsu Province Department of Health.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Cancer.gov
Entrez
LocusLink
FURTHER INFORMATION
International Agency for Research on Cancer
Medline plus — hepatocellular carcinoma
World Health Organisation — hepatitis B
US Food & Drug Administration — aflatoxins
Glossary
- MYCOTOXINS
-
A group of chemically diverse secondary fungal metabolites that can induce various toxic responses in humans and animals when food containing these compounds is ingested. More than 100 structurally characterized mycotoxins are known.
- SERUM TRANSAMINASES
-
These are used to detect liver injury, particularly to the parenchymal cells. Two commonly measured serum enzymes are aspartate aminotransferase (AST), which is somewhat nonspecific in that it can also indicate injury to non-hepatic tissues, and alanine aminotransferase (ALT), which is mainly found in the liver and is released during hepatotoxicity.
- SCHISTOSOMIASIS
-
A severe, debilitating disease caused by infection with trematodes of the genus Schistosoma. Infection in humans is caused by cercaria, which are liberated from the invertebrate host (snails). They penetrate the skin and mainly affect the genitourinary and gastrointestinal systems.
- ISOTHIOCYANATES
-
These exist in plants as relatively stable β-thioglucoside N-hydroxysulphate precursor conjugates (known as glucosinolates). The enzyme myrosinase promotes the hydrolysis of glucosinolates to isothiocyanates. Myrosinase is normally physically segregated from its glucosinolate substrates, but it is released when plant cells are injured (for example, by chewing, food preparation or insect predation). This reaction is responsible for the bitter taste of horseradish, mustard and wasabi.
Rights and permissions
About this article
Cite this article
Kensler, T., Qian, GS., Chen, JG. et al. Translational strategies for cancer prevention in liver. Nat Rev Cancer 3, 321–329 (2003). https://doi.org/10.1038/nrc1076
Issue Date:
DOI: https://doi.org/10.1038/nrc1076