Key Points
-
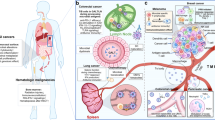
The human microbiota is the ensemble of bacteria and other microorganisms that inhabit the epithelial barrier surfaces of the body. The microbiota affects physiological functions, particularly metabolism, neurological and cognitive functions, haematopoiesis, inflammation and immunity.
-
The microbiota and its larger host represent a metaorganism in which the crosstalk between microorganisms and host cells is necessary for health, survival and regulation of physiological functions at the local barrier level and systemically. Mostly because of its effects on metabolism, cellular proliferation, inflammation and immunity, the microbiota regulates cancer at the level of predisposing conditions, initiation, genetic instability, susceptibility to host immune response, progression, comorbidity and response to therapy.
-
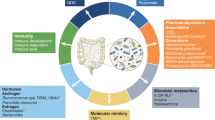
The gut microbiota affects aspects of drug metabolism, pharmacokinetics, anticancer effect and toxicity. The rate of absorption and bioavailability of many oral drugs, including cancer therapies, depends on their exposure in the gut to both host and bacterial enzymes before entering the circulation.
-
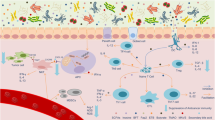
The microbiota regulates the response to different types of cancer chemotherapy by affecting their mechanism of action and toxicity. The best characterized are oxaliplatin and cyclophosphamide; the anticancer activity of which is affected by the gut microbiota, which primes myeloid cells for production of reactive oxygen species in the case of oxaliplatin and facilitates the induction of an anticancer T cell response in the case of CTX.
-
The role of the microbiota in modulating the response to anticancer radiotherapy remains to be fully characterized. However, germ-free mice have been described as being less susceptible to the toxicity of radiation than conventionally raised mice, and evidence in humans and experimental animals suggests that the composition of the intestinal microbiota may affect the severity of radiation-induced mucosal toxicity.
-
The composition of the gut microbiota modulates both inflammation and adaptive immunity and thereby regulates the effectiveness of cancer immune therapies, such as adoptive T cell transfer preceded by total body irradiation, intratumoural treatment with CpG-oligodeoxynucleotides and immune checkpoint blockade with anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA4) and anti-programmed cell death protein 1 ligand 1 (PDL1).
-
Evidence of the important role of the microbiota in controlling cancer therapy effectiveness and toxicity is derived mainly from data in experimental animals, and translation of these findings to human clinical medicine remains challenging. Additional human data should be obtained and new technologies developed in order to safely target the microbiota to improve anticancer therapies while attenuating the toxic side effects.
Abstract
The microbiota is composed of commensal bacteria and other microorganisms that live on the epithelial barriers of the host. The commensal microbiota is important for the health and survival of the organism. Microbiota influences physiological functions from the maintenance of barrier homeostasis locally to the regulation of metabolism, haematopoiesis, inflammation, immunity and other functions systemically. The microbiota is also involved in the initiation, progression and dissemination of cancer both at epithelial barriers and in sterile tissues. Recently, it has become evident that microbiota, and particularly the gut microbiota, modulates the response to cancer therapy and susceptibility to toxic side effects. In this Review, we discuss the evidence for the ability of the microbiota to modulate chemotherapy, radiotherapy and immunotherapy with a focus on the microbial species involved, their mechanism of action and the possibility of targeting the microbiota to improve anticancer efficacy while preventing toxicity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
04 April 2017
In this article the sentence 'however, in one study, overgrowth of Parabacteroides distasonis in mice treated with broad-spectrum antibiotics was observed to abrogate its antitumour effect' was incorrectly referenced. The correct reference for this sentence is 61.
References
Costello, E. K., Stagaman, K., Dethlefsen, L., Bohannan, B. J. & Relman, D. A. The application of ecological theory toward an understanding of the human microbiome. Science 336, 1255–1262 (2012).
Bosch, T. C. & McFall-Ngai, M. J. Metaorganisms as the new frontier. Zoology (Jena) 114, 185–190 (2011).
Dzutsev, A., Goldszmid, R. S., Viaud, S., Zitvogel, L. & Trinchieri, G. The role of the microbiota in inflammation, carcinogenesis, and cancer therapy. Eur. J. Immunol. 45, 17–31 (2015).
Smith, K., McCoy, K. D. & Macpherson, A. J. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin. Immunol. 19, 59–69 (2007).
Gustafsson, B. E., Daft, F. S., McDaniel, E. G., Smith, J. C. & Fitzgerald, R. J. Effects of vitamin K-active compounds and intestinal microorganisms in vitamin K-deficient germfree rats. J. Nutr. 78, 461–468 (1962).
Gordon, H. A., Bruckner-Kardoss, E. & Wostmann, B. S. Aging in germ-free mice: life tables and lesions observed at natural death. J. Gerontol. 21, 380–387 (1966).
De Santis, S., Cavalcanti, E., Mastronardi, M., Jirillo, E. & Chieppa, M. Nutritional keys for intestinal barrier modulation. Front. Immunol. 6, 612 (2015).
Vaishnava, S., Behrendt, C. L., Ismail, A. S., Eckmann, L. & Hooper, L. V. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host–microbial interface. Proc. Natl Acad. Sci. USA 105, 20858 (2008). This paper identified the role of microbiota signalling in maintaining the host–commensal homeostasis through MYD88-coupled receptors in epithelial cells.
Peterson, L. W. & Artis, D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 14, 141–153 (2014).
Belkaid, Y. & Naik, S. Compartmentalized and systemic control of tissue immunity by commensals. Nat. Immunol. 14, 646–653 (2013). This review discusses the role of the microbiota at different epithelial barriers in regulating immunity both locally and systemically.
Belkaid, Y. & Hand, T. W. Role of the microbiota in immunity and inflammation. Cell 157, 121–141 (2014).
Sender, R., Fuchs, S. & Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 14, e1002533 (2016).
Chow, J., Tang, H. & Mazmanian, S. K. Pathobionts of the gastrointestinal microbiota and inflammatory disease. Curr. Opin. Immunol. 23, 473–480 (2011).
Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015).
Khosravi, A. et al. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe 15, 374–381 (2014).
Trompette, A. et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 20, 159–166 (2014).
Chung, W. S. F. et al. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol. 14, 3 (2016).
Dinan, T. G. & Cryan, J. F. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology 37, 1369–1378 (2012).
Sommer, F. & Bäckhed, F. The gut microbiota engages different signaling pathways to induce Duox2 expression in the ileum and colon epithelium. Mucosal Immunol. 8, 372–379 (2015).
Wells, J. M., Rossi, O., Meijerink, M. & van Baarlen, P. Epithelial crosstalk at the microbiota–mucosal interface. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4607–4614 (2011).
Tulstrup, M. V.-L. et al. Antibiotic treatment affects intestinal permeability and gut microbial composition in Wistar rats dependent on antibiotic class. PLoS ONE 10, e0144854 (2015).
Backhed, F. et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703 (2015).
Dominguez-Bello, M. G. et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010). References 22 and 23 describe the establishment of the human microbiota during early life.
Faith, J. J. et al. The long-term stability of the human gut microbiota. Science 341, 1237439 (2013).
Oh, J., Byrd, A. L., Park, M., Kong, H. H. & Segre, J. A. Temporal stability of the human skin microbiome. Cell 165, 854–866 (2016).
Goodrich, J. K. et al. Human genetics shape the gut microbiome. Cell 159, 789–799 (2014).
Turnbaugh, P. J. et al. A core gut microbiome in obese and lean twins. Nature 457, 480–484 (2009).
David, L. A. et al. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 15, R89 (2014).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Garrett, W. S. et al. Colitis-associated colorectal cancer driven by T-bet deficiency in dendritic cells. Cancer Cell 16, 208–219 (2009).
Couturier-Maillard, A. et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J. Clin. Invest. 123, 700–711 (2013).
Hu, B. et al. Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proc. Natl Acad. Sci. USA 110, 9862–9867 (2013). References 31 and 32 demonstrated that the carcinogenic phenotypes associated with dysbiosis of the microbiota in genetically mutated mice can be transmitted to wild-type mice by microbiota transfer.
Vetizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015). This paper presented the first demonstration of the role of the microbiota in modulating responsiveness to anti-CTLA4 therapy.
DeVita, V. T. Jr & Chu, E. A history of cancer chemotherapy. Cancer Res. 68, 8643–8653 (2008).
Sancho-Martinez, S. M., Prieto-Garcia, L., Prieto, M., Lopez-Novoa, J. M. & Lopez-Hernandez, F. J. Subcellular targets of cisplatin cytotoxicity: an integrated view. Pharmacol. Ther. 136, 35–55 (2012).
Mitchell, E. P. Gastrointestinal toxicity of chemotherapeutic agents. Semin. Oncol. 33, 106–120 (2006).
Spanogiannopoulos, P., Bess, E. N., Carmody, R. N. & Turnbaugh, P. J. The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nat. Rev. Microbiol. 14, 273–287 (2016).
Li, H. & Jia, W. Cometabolism of microbes and host: implications for drug metabolism and drug-induced toxicity. Clin. Pharmacol. Ther. 94, 574–581 (2013).
Feng, R. et al. Transforming berberine into its intestine-absorbable form by the gut microbiota. Sci. Rep. 5, 12155 (2015).
Maurice, C. F., Haiser, H. J. & Turnbaugh, P. J. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell 152, 39–50 (2013).
Montassier, E. et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 42, 515–528 (2015).
Wilson, I. D. & Nicholson, J. K. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res. 179, 204–222 (2017).
Haiser, H. J. & Turnbaugh, P. J. Developing a metagenomic view of xenobiotic metabolism. Pharmacol. Res. 69, 21–31 (2013).
Carmody, R. N. & Turnbaugh, P. J. Host–microbial interactions in the metabolism of therapeutic and diet-derived xenobiotics. J. Clin. Invest. 124, 4173–4181 (2014).
Bjorkholm, B. et al. Intestinal microbiota regulate xenobiotic metabolism in the liver. PLoS ONE 4, e6958 (2009).
Selwyn, F. P., Cheng, S. L., Klaassen, C. D. & Cui, J. Y. Regulation of hepatic drug-metabolizing enzymes in germ-free mice by conventionalization and probiotics. Drug Metab. Dispos. 44, 262–274 (2016).
Selwyn, F. P., Cui, J. Y. & Klaassen, C. D. RNA-Seq quantification of hepatic drug processing genes in germ-free mice. Drug Metab. Dispos. 43, 1572–1580 (2015).
Selwyn, F. P. et al. Developmental regulation of drug-processing genes in livers of germ-free mice. Toxicol. Sci. 147, 84–103 (2015).
Kang, M. J. et al. The effect of gut microbiota on drug metabolism. Expert Opin. Drug Metab. Toxicol. 9, 1295–1308 (2013).
Yip, L. Y. & Chan, E. C. Investigation of host-gut microbiota modulation of therapeutic outcome. Drug Metab. Dispos. 43, 1619–1631 (2015).
Fujita, K. & Sparreboom, A. Pharmacogenetics of irinotecan disposition and toxicity: a review. Curr. Clin. Pharmacol. 5, 209–217 (2010).
Stringer, A. M. et al. Faecal microflora and beta-glucuronidase expression are altered in an irinotecan-induced diarrhea model in rats. Cancer Biol. Ther. 7, 1919–1925 (2008).
Lin, X. B. et al. Irinotecan (CPT-11) chemotherapy alters intestinal microbiota in tumour bearing rats. PLoS ONE 7, e39764 (2012).
Dabek, M., McCrae, S. I., Stevens, V. J., Duncan, S. H. & Louis, P. Distribution of beta-glucosidase and beta-glucuronidase activity and of beta-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol. Ecol. 66, 487–495 (2008).
McIntosh, F. M. et al. Phylogenetic distribution of genes encoding beta-glucuronidase activity in human colonic bacteria and the impact of diet on faecal glycosidase activities. Environ. Microbiol. 14, 1876–1887 (2012).
Takasuna, K. et al. Involvement of beta-glucuronidase in intestinal microflora in the intestinal toxicity of the antitumor camptothecin derivative irinotecan hydrochloride (CPT-11) in rats. Cancer Res. 56, 3752–3757 (1996).
Wallace, B. D. et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 330, 831–835 (2010).
Mego, M. et al. Prevention of irinotecan induced diarrhea by probiotics: a randomized double blind, placebo controlled pilot study. Complement. Ther. Med. 23, 356–362 (2015).
Wallace, B. D. et al. Structure and inhibition of microbiome beta-glucuronidases essential to the alleviation of cancer drug toxicity. Chem. Biol. 22, 1238–1249 (2015).
Lehouritis, P. et al. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci. Rep. 5, 14554 (2015).
Viaud, S. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976 (2013). This paper describes the role of the microbiota in modulating the anticancer effect of CTX.
Iida, N. et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970 (2013). This paper describes the role of the microbiota in modulating the efficacy of cancer therapy with CpG-ODNs and platinum drugs.
Galluzzi, L. et al. Systems biology of cisplatin resistance: past, present and future. Cell Death Dis. 5, e1257 (2014).
Roy, S., Ryals, M. M., Van den Bruele, A. B., Fitzgerald, T. S. & Cunningham, L. L. Sound preconditioning therapy inhibits ototoxic hearing loss in mice. J. Clin. Invest. 123, 4945–4949 (2013).
Abuzeid, W. M. et al. Molecular disruption of RAD50 sensitizes human tumor cells to cisplatin-based chemotherapy. J. Clin. Invest. 119, 1974–1985 (2009).
Pabla, N. & Dong, Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 73, 994–1007 (2008).
Wagner, J. M. & Karnitz, L. M. Cisplatin-induced DNA damage activates replication checkpoint signaling components that differentially affect tumor cell survival. Mol. Pharmacol. 76, 208–214 (2009).
Zhu, S., Pabla, N., Tang, C., He, L. & Dong, Z. DNA damage response in cisplatin-induced nephrotoxicity. Arch. Toxicol. 89, 2197–2205 (2015).
Park, S. B. et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J. Clin. 63, 419–437 (2013).
Hooper, L. V. & Macpherson, A. J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 10, 159–169 (2010).
Sonis, S. T. The pathobiology of mucositis. Nat. Rev. Cancer 4, 277–284 (2004).
Kim, S., Lee, T. J., Park, J. W. & Kwon, T. K. Overexpression of cFLIPs inhibits oxaliplatin-mediated apoptosis through enhanced XIAP stability and Akt activation in human renal cancer cells. J. Cell. Biochem. 105, 971–979 (2008).
Laurent, A. et al. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 65, 948–956 (2005).
Gui, Q. F., Lu, H. F., Zhang, C. X., Xu, Z. R. & Yang, Y. H. Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet. Mol. Res. 14, 5642–5651 (2015).
Tesniere, A. et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 29, 482–491 (2010).
Michaud, M. et al. Subversion of the chemotherapy-induced anticancer immune response by the ecto-ATPase CD39. Oncoimmunology 1, 393–395 (2012).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 (2009).
Pateras, I. S. et al. The DNA damage response and immune signaling alliance: is it good or bad? Nature decides when and where. Pharmacol. Ther. 154, 36–56 (2015).
Vacchelli, E. et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science 350, 972–978 (2015).
Kroemer, G., Galluzzi, L., Kepp, O. & Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31, 51–72 (2013). This paper reviewed the mechanisms underlying the phenomenon of immunogenic cell death in cancer therapy.
Zwielehner, J. et al. Changes in human fecal microbiota due to chemotherapy analyzed by TaqMan-PCR, 454 sequencing and PCR-DGGE fingerprinting. PLoS ONE 6, e28654 (2011).
Daillere, R. et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 45, 931–943 (2016). This paper characterized the role of different bacterial species in regulating antitumour T cell responses induced by CTX.
Ghoreschi, K. et al. Generation of pathogenic TH17 cells in the absence of TGF-beta signalling. Nature 467, 967–971 (2010).
Chitapanarux, I. et al. Randomized controlled trial of live Lactobacillus acidophilus plus Bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat. Oncol. 5, 31 (2010).
Wang, Y. et al. Pharmacological inhibition of NADPH oxidase protects against cisplatin induced nephrotoxicity in mice by two step mechanism. Food Chem. Toxicol. 83, 251–260 (2015).
Cario, E. Toll-like receptors in the pathogenesis of chemotherapy-induced gastrointestinal toxicity. Curr. Opin. Support. Palliat. Care 10, 157–164 (2016).
Frank, M. et al. TLR signaling modulates side effects of anticancer therapy in the small intestine. J. Immunol. 194, 1983–1995 (2015).
Mercado-Lubo, R. & McCormick, B. A. The interaction of gut microbes with host ABC transporters. Gut Microbes 1, 301–306 (2010).
Napenas, J. J. et al. Molecular methodology to assess the impact of cancer chemotherapy on the oral bacterial flora: a pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 109, 554–560 (2010).
Niu, Q. Y., Li, Z. Y., Du, G. H. & Qin, X. M. 1H NMR based metabolomic profiling revealed doxorubicin-induced systematic alterations in a rat model. J. Pharm. Biomed. Anal. 118, 338–348 (2016).
Rigby, R. J. et al. Intestinal bacteria are necessary for doxorubicin-induced intestinal damage but not for doxorubicin-induced apoptosis. Gut Microbes 7, 414–423 (2016).
Nigro, G., Rossi, R., Commere, P. H., Jay, P. & Sansonetti, P. J. The cytosolic bacterial peptidoglycan sensor Nod2 affords stem cell protection and links microbes to gut epithelial regeneration. Cell Host Microbe 15, 792–798 (2014).
Jiang, C. et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat. Commun. 6, 10166 (2015).
Parseus, A. et al. Microbiota-induced obesity requires farnesoid X receptor. Gut 66, 429–437 (2016).
Das, S. K. et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 333, 233–238 (2011).
Ruud, J. et al. Inflammation- and tumor-induced anorexia and weight loss require MyD88 in hematopoietic/myeloid cells but not in brain endothelial or neural cells. FASEB J. 27, 1973–1980 (2013).
Suárez-Zamorano, N. et al. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat. Med. 21, 1497–1501 (2015).
de Matos-Neto, E. M. et al. Systemic inflammation in cachexia — is tumor cytokine expression profile the culprit? Front. Immunol. 6, 629 (2015).
Antoun, S., Baracos, V. E., Birdsell, L., Escudier, B. & Sawyer, M. B. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann. Oncol. 21, 1594–1598 (2010).
Toledo, M. et al. A multifactorial anti-cachectic approach for cancer cachexia in a rat model undergoing chemotherapy. J. Cachexia Sarcopenia Muscle 7, 48–59 (2016).
Conte, E. et al. Cisplatin-induced cachexia in rats causes alterations in skeletal muscle calcium homeostasis. Biophys. J. 108 (Suppl. 1), 108a (2015).
Garcia, J. M., Cata, J. P., Dougherty, P. M. & Smith, R. G. Ghrelin prevents cisplatin-induced mechanical hyperalgesia and cachexia. Endocrinology 149, 455–460 (2008).
Bruggeman, A. R. et al. Cancer cachexia: beyond weight loss. J. Oncol. Pract. 12, 1163–1171 (2016).
Cvan Trobec, K. et al. Influence of cancer cachexia on drug liver metabolism and renal elimination in rats. J. Cachexia Sarcopenia Muscle 6, 45–52 (2015).
Bindels, L. B. & Delzenne, N. M. Muscle wasting: the gut microbiota as a new therapeutic target? Int. J. Biochem. Cell Biol. 45, 2186–2190 (2013).
Klein, G. L., Petschow, B. W., Shaw, A. L. & Weaver, E. Gut barrier dysfunction and microbial translocation in cancer cachexia: a new therapeutic target. Curr. Opin. Support. Palliat. Care 7, 361–367 (2013).
Yeh, K. Y. et al. Omega-3 fatty acid-, micronutrient-, and probiotic-enriched nutrition helps body weight stabilization in head and neck cancer cachexia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 116, 41–48 (2013).
Varian, B. J. et al. Beneficial bacteria inhibit cachexia. Oncotarget 7, 11803–11816 (2016).
Schieber, A. M. P. et al. Disease tolerance mediated by microbiome E. coli involves inflammasome and IGF-1 signaling. Science 350, 558–563 (2015).
Mavragani, I. V. et al. Key mechanisms involved in ionizing radiation-induced systemic effects. A current review. Toxicol. Res. 5, 12–33 (2016).
Azzam, E. I. & Little, J. B. The radiation-induced bystander effect: evidence and significance. Hum. Exp. Toxicol. 23, 61–65 (2004).
Vacchelli, E. et al. Trial Watch: anticancer radioimmunotherapy. Oncoimmunology 2, e25595 (2013).
Apetoh, L. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 13, 1050–1059 (2007).
Nikitaki, Z. et al. Systemic mechanisms and effects of ionizing radiation: a new 'old' paradigm of how the bystanders and distant can become the players. Semin. Cancer Biol. 37–38, 77–95 (2016).
Ermolaeva, M. A. et al. DNA damage in germ cells induces an innate immune response that triggers systemic stress resistance. Nature 501, 416–420 (2013).
Al-Mayah, A. et al. The non-targeted effects of radiation are perpetuated by exosomes. Mutat. Res. 772, 38–45 (2015).
Demaria, S. & Formenti, S. C. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front. Oncol. 2, 153 (2012).
Demaria, S. et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 58, 862–870 (2004).
Zitvogel, L., Ayyoub, M., Routy, B. & Kroemer, G. Microbiome and anticancer immunosurveillance. Cell 165, 276–287 (2016).
Deng, L. et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Invest. 124, 687–695 (2014).
Baird, J. R. et al. Radiotherapy combined with novel STING-targeting oligonucleotides results in regression of established tumors. Cancer Res. 76, 50–61 (2016).
Barker, H. E., Paget, J. T. E., Khan, A. A. & Harrington, K. J. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat. Rev. Cancer 15, 409–425 (2015).
Touchefeu, Y. et al. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis — current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 40, 409–421 (2014).
Vanhoecke, B. W. et al. Low-dose irradiation affects the functional behavior of oral microbiota in the context of mucositis. Exp. Biol. Med. (Maywood) 241, 60–70 (2016).
Broin, P. Ó. et al. Intestinal microbiota-derived metabolomic blood plasma markers for prior radiation injury. Int. J. Radiat. Oncol. Biol. Phys. 91, 360–367 (2015).
Wang, A. et al. Gut microbial dysbiosis may predict diarrhea and fatigue in patients undergoing pelvic cancer radiotherapy: a pilot study. PLoS ONE 10, e0126312 (2015).
Takemura, N. et al. Blockade of TLR3 protects mice from lethal radiation-induced gastrointestinal syndrome. Nat. Commun. 5, 3492 (2014).
Vacchelli, E. et al. Trial Watch: Toll-like receptor agonists for cancer therapy. Oncoimmunology 2, e25238 (2013).
Hu, B. et al. The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science 354, 765–768 (2016).
Ciorba, M. A. et al. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut 61, 829–838 (2012).
Jones, R. M. et al. Lactobacilli modulate epithelial cytoprotection through the Nrf2 pathway. Cell Rep. 12, 1217–1225 (2015).
Jones, R. M. et al. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. EMBO J. 32, 3017–3028 (2013).
Delia, P. et al. Use of probiotics for prevention of radiation-induced diarrhea. World J. Gastroenterol. 13, 912–915 (2007).
Sharma, A. et al. Lactobacillus brevis CD2 lozenges reduce radiation- and chemotherapy-induced mucositis in patients with head and neck cancer: a randomized double-blind placebo-controlled study. Eur. J. Cancer 48, 875–881 (2012).
Crawford, P. A. & Gordon, J. I. Microbial regulation of intestinal radiosensitivity. Proc. Natl Acad. Sci. USA 102, 13254–13259 (2005). This paper describes the radioresistance of germ-free mice and characterized the underlying molecular mechanisms.
Santulli, G. Angiopoietin-like proteins: a comprehensive look. Front. Endocrinol. 5, 4 (2014).
Grootaert, C. et al. Bacterial monocultures, propionate, butyrate and H2O2 modulate the expression, secretion and structure of the fasting-induced adipose factor in gut epithelial cell lines. Environ. Microbiol. 13, 1778–1789 (2011).
Jacouton, E. et al. Lactobacillus rhamnosus CNCMI-4317 modulates Fiaf/Angptl4 in intestinal epithelial cells and circulating level in mice. PLoS ONE 10, e0138880 (2015).
Korecka, A. et al. ANGPTL4 expression induced by butyrate and rosiglitazone in human intestinal epithelial cells utilizes independent pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G1025–G1037 (2013).
Duncan, A. M., Ronen, A. & Blakey, D. H. Diurnal variation in the response of gamma-ray-induced apoptosis in the mouse intestinal epithelium. Cancer Lett. 21, 163–166 (1983).
Ishihara, H. et al. Circadian transitions in radiation dose-dependent augmentation of mRNA levels for DNA damage-induced genes elicited by accurate real-time RT-PCR quantification. J. Radiat. Res. 51, 265–275 (2010).
Ruifrok, A. C., Weil, M. M., Thames, H. D. & Mason, K. A. Diurnal variations in the expression of radiation-induced apoptosis. Radiat. Res. 149, 360–365 (1998).
Leone, V. et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 17, 681–689 (2015).
Liang, X., Bushman, F. D. & FitzGerald, G. A. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc. Natl Acad. Sci. USA 112, 10479–10484 (2015).
Mukherji, A., Kobiita, A., Ye, T. & Chambon, P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 153, 812–827 (2013).
Nguyen, K. D. et al. Circadian gene Bmal1 regulates diurnal oscillations of Ly6Chi inflammatory monocytes. Science 341, 1483–1488 (2013).
Thaiss, C. A. et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159, 514–529 (2014).
Maier, I., Berry, D. M. & Schiestl, R. H. Intestinal microbiota reduces genotoxic endpoints induced by high-energy protons. Radiat. Res. 181, 45–53 (2014).
Holohan, C., Van Schaeybroeck, S., Longley, D. B. & Johnston, P. G. Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer 13, 714–726 (2013).
Couzin-Frankel, J. Breakthrough of the year 2013. Cancer immunotherapy. Science 342, 1432–1433 (2013).
Mellman, I., Coukos, G. & Dranoff, G. Cancer immunotherapy comes of age. Nature 480, 480–489 (2011).
Sivan, A. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015). This paper demonstrated that the presence of Bifidobacterium spp. in the gut microbiota promotes antitumour immunity in mice that is amplified by anti-PDL1 therapy.
Paulos, C. M. et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Invest. 117, 2197–2204 (2007). This paper used a therapy model of adoptive T cell transfer preceded by TBI in mice, to demonstrate for the first time that the microbiota modulates anticancer therapy.
Dudley, M. E. et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 26, 5233–5239 (2008).
Guiducci, C., Vicari, A. P., Sangaletti, S., Trinchieri, G. & Colombo, M. P. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 65, 3437–3446 (2005).
Vicari, A. P. et al. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J. Exp. Med. 196, 541–549 (2002).
Stewart, C. A. et al. Interferon-dependent IL-10 production by Tregs limits tumor Th17 inflammation. J. Clin. Invest. 123, 4859–4874 (2013).
Netea, M. G. et al. Trained immunity: a program of innate immune memory in health and disease. Science 352, aaf1098 (2016).
Rosenberg, S. A., Yang, J. C. & Restifo, N. P. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 10, 909–915 (2004).
Eggermont, A. M. Therapeutic vaccines in solid tumours: can they be harmful? Eur. J. Cancer 45, 2087–2090 (2009).
Page, D. B., Postow, M. A., Callahan, M. K., Allison, J. P. & Wolchok, J. D. Immune modulation in cancer with antibodies. Annu. Rev. Med. 65, 185–202 (2014).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Teply, B. A. & Lipson, E. J. Identification and management of toxicities from immune checkpoint-blocking drugs. Oncology (Williston Park) 28 (Suppl. 3), 30–38 (2014).
Dubin, K. et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 7, 10391 (2016).
Hand, T. W. et al. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science 337, 1553–1556 (2012).
Yang, X. et al. Targeting the tumor microenvironment with interferon-β bridges innate and adaptive immune responses. Cancer Cell 25, 37–48 (2014).
Zitvogel, L., Pitt, J. M., Daillere, R., Smyth, M. J. & Kroemer, G. Mouse models in oncoimmunology. Nat. Rev. Cancer 16, 759–773 (2016).
Ivanov, I. I., Frutos Rde, L., Manel, N., Yoshinaga, K. & Rifkin, D. B. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4, 337 (2008).
Nowarski, R. et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell 163, 1444–1456 (2015).
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 (2011).
Wheeler, M. L. et al. Immunological consequences of intestinal fungal dysbiosis. Cell Host Microbe 19, 865–873 (2016).
Howitt, M. R. et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351, 1329–1333 (2016).
Kernbauer, E., Ding, Y. & Cadwell, K. An enteric virus can replace the beneficial function of commensal bacteria. Nature 516, 94–98 (2014).
Cadwell, K. The virome in host health and disease. Immunity 42, 805–813 (2015).
Ramanan, D. et al. Helminth infection promotes colonization resistance via type 2 immunity. Science 352, 608–612 (2016).
Young, G. R. et al. Resurrection of endogenous retroviruses in antibody-deficient mice. Nature 491, 774–778 (2012).
Turnbaugh, P. J. et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci. Transl Med. 1, 6ra14 (2009).
Baxter, N. T., Zackular, J. P., Chen, G. Y. & Schloss, P. D. Structure of the gut microbiome following colonization with human feces determines colonic tumor burden. Microbiome 2, 20 (2014).
Plantinga, T. S. et al. Differential Toll-like receptor recognition and induction of cytokine profile by Bifidobacterium breve and Lactobacillus strains of probiotics. Clin. Vaccine Immunol. 18, 621–628 (2011).
Kadowaki, N. et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194, 863–869 (2001).
Pamer, E. G. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science 352, 535–538 (2016).
Goldszmid, R. S. & Trinchieri, G. The price of immunity. Nat. Immunol. 13, 932–938 (2012).
Jobin, C. Colorectal cancer: CRC — all about microbial products and barrier function? Nat. Rev. Gastroenterol. Hepatol. 9, 694–696 (2012).
Rao, V. P. et al. Innate immune inflammatory response against enteric bacteria Helicobacter hepaticus induces mammary adenocarcinoma in mice. Cancer Res. 66, 7395–7400 (2006).
Salcedo, R. et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J. Exp. Med. 207, 1625–1636 (2010).
Allen, I. C. et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 207, 1045–1056 (2010).
Sears, C. L. & Garrett, W. S. Microbes, microbiota, and colon cancer. Cell Host Microbe 15, 317–328 (2014).
Kostic, A. D. et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14, 207–215 (2013).
Rubinstein, M. R. et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 14, 195–206 (2013).
Gur, C. et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 42, 344–355 (2015).
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Schistosomes, liver flukes and Helicobacter pylori. Lyon, 7–14 June 1994. IARC Monogr. Eval. Carcinog. Risks Hum. 61, 1–241 (1994).
Poutahidis, T. et al. Pathogenic intestinal bacteria enhance prostate cancer development via systemic activation of immune cells in mice. PLoS ONE 8, e73933 (2013).
Fox, J. G. et al. Gut microbes define liver cancer risk in mice exposed to chemical and viral transgenic hepatocarcinogens. Gut 59, 88–97 (2010).
Yamamoto, M. L. et al. Intestinal bacteria modify lymphoma incidence and latency by affecting systemic inflammatory state, oxidative stress, and leukocyte genotoxicity. Cancer Res. 73, 4222–4232 (2013).
Farrell, J. J. et al. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 61, 582–588 (2012).
Fan, X. et al. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut http://dx.doi.org/10.1136/gutjnl-2016-312580 (2016).
Westbrook, A. M. et al. The role of tumour necrosis factor-alpha and tumour necrosis factor receptor signalling in inflammation-associated systemic genotoxicity. Mutagenesis 27, 77–86 (2012).
Gyurkocza, B., Rezvani, A. & Storb, R. F. Allogeneic hematopoietic cell transplantation: the state of the art. Expert Rev. Hematol. 3, 285–299 (2010).
Taur, Y., Jenq, R. R., Ubeda, C., van den Brink, M. & Pamer, E. G. Role of intestinal microbiota in transplantation outcomes. Best Pract. Res. Clin. Haematol. 28, 155–161 (2015).
Taur, Y. et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 124, 1174–1182 (2014).
Jenq, R. R. et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J. Exp. Med. 209, 903–911 (2012).
Holler, E. et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol. Blood Marrow Transplant. 20, 640–645 (2014).
Taur, Y. et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin. Infect. Dis. 55, 905–914 (2012).
Jenq, R. R. et al. Intestinal blautia is associated with reduced death from graft-versus-host disease. Biol. Blood Marrow Transplant. 21, 1373–1383 (2015).
Ho, J. T. K., Chan, G. C. F. & Li, J. C. B. Systemic effects of gut microbiota and its relationship with disease and modulation. BMC Immunol. 16, 21 (2015).
Boleij, A. et al. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin. Infect. Dis. 60, 208–215 (2015).
Koshiol, J. et al. Salmonella enterica serovar Typhi and gallbladder cancer: a case-control study and meta-analysis. Cancer Med. 5, 3310–3235 (2016).
Lecuit, M. et al. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N. Engl. J. Med. 350, 239–248 (2004).
Senff, N. J. et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood 112, 1600–1609 (2008).
Ferreri, A. J. et al. Chlamydophila psittaci eradication with doxycycline as first-line targeted therapy for ocular adnexae lymphoma: final results of an international phase II trial. J. Clin. Oncol. 30, 2988–2994 (2012).
Lakritz, J. R. et al. Gut bacteria require neutrophils to promote mammary tumorigenesis. Oncotarget 6, 9387–9396 (2015).
Rutkowski, M. R. et al. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell 27, 27–40 (2015).
Chu, H. & Mazmanian, S. K. Innate immune recognition of the microbiota promotes host–microbial symbiosis. Nat. Immunol. 14, 668–675 (2013).
McFall-Ngai, M. et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl Acad. Sci. USA 110, 3229–3236 (2013).
Kremer, N. et al. Initial symbiont contact orchestrates host-organ-wide transcriptional changes that prime tissue colonization. Cell Host Microbe 14, 183–194 (2013).
Hansen, C. H. et al. Patterns of early gut colonization shape future immune responses of the host. PLoS ONE 7, e34043 (2012).
Stappenbeck, T. S., Hooper, L. V. & Gordon, J. I. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc. Natl Acad. Sci. USA 99, 15451–15455 (2002).
Le, Y. et al. Biologically active peptides interacting with the G protein-coupled formylpeptide receptor. Protein Pept. Lett. 14, 846–853 (2007).
Chen, K. et al. Formylpeptide receptor-2 contributes to colonic epithelial homeostasis, inflammation, and tumorigenesis. J. Clin. Invest. 123, 1694–1704 (2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Germ-free animals
-
Animals raised in strict sterile conditions that have no microorganisms living in or on them.
- Commensalism
-
A symbiotic relationship between two species in which one species benefits without causing harm to the other.
- Pathobionts
-
Resident commensal microorganisms that under certain conditions may acquire pathogenic potential.
- Mutualism
-
A symbiotic relationship between two species that is beneficial for both species.
- Xenobiotics
-
Foreign chemical substances, including drugs, that are not naturally produced by the organism.
- Biotransformation
-
The chemical alteration of a xenobiotic, such as a drug, within the body.
- Probiotic
-
Live microorganisms that are consumed by humans and animals as food supplements for their potential health-promoting qualities.
- Pathogenic T helper 17 cells
-
(pTH17 cells). A CD4+ T cell subset that simultaneously expresses markers of TH1 cells (T-bet transcription factor, interferon–γ (IFN–γ) and CXC chemokine receptor 3 (CXCR3)) and of TH17 cells (RORγT transcription factor, interleukin-17 (IL-17) and C-C chemokine receptor 6 (CCR6)).
- Cachexia
-
A wasting syndrome with muscle atrophy and loss of weight and adipose tissue, often associated with cancer and cancer therapy.
- Bystander effect
-
In radiobiology, collateral damage exhibited by unirradiated cells in response to signals received from nearby irradiated cells.
- Abscopal effect
-
In radiotherapy, a phenomenon whereby local radiotherapy induces tumour regression at sites distant from the irradiated site.
- Prebiotics
-
Non-digestible food ingredients, often containing fibre, that promote the growth of beneficial microorganisms in the intestines.
- Enterotypes
-
Classification of individuals based on the composition of their gut bacterial ecosystem, each enterotype having distinct clusters of organisms with characteristic predominant bacterial species.
Rights and permissions
About this article
Cite this article
Roy, S., Trinchieri, G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer 17, 271–285 (2017). https://doi.org/10.1038/nrc.2017.13
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc.2017.13
This article is cited by
-
Microbiome in radiotherapy: an emerging approach to enhance treatment efficacy and reduce tissue injury
Molecular Medicine (2024)
-
Strategies to enhance the therapeutic efficacy of anti-PD-1 antibody, anti-PD-L1 antibody and anti-CTLA-4 antibody in cancer therapy
Journal of Translational Medicine (2024)
-
Drug repurposing for cancer therapy
Signal Transduction and Targeted Therapy (2024)
-
Gut microbial metabolite facilitates colorectal cancer development via ferroptosis inhibition
Nature Cell Biology (2024)
-
Lung microbiome: new insights into the pathogenesis of respiratory diseases
Signal Transduction and Targeted Therapy (2024)