Abstract
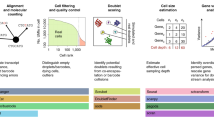
Measurement of the transcriptomes of single cells has been feasible for only a few years, but it has become an extremely popular assay. While many types of analysis can be carried out and various questions can be answered by single-cell RNA-seq, a central focus is the ability to survey the diversity of cell types in a sample. Unbiased and reproducible cataloging of gene expression patterns in distinct cell types requires large numbers of cells. Technological developments and protocol improvements have fueled consistent and exponential increases in the number of cells that can be studied in single-cell RNA-seq analyses. In this Perspective, we highlight the key technological developments that have enabled this growth in the data obtained from single-cell RNA-seq experiments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Change history
22 March 2018
In the version of this article initially published, the sentence "This method was also used for MARS-seq (massively parallel scRNA-seq)33, and later for InDrop (droplet sequencing)34 and Seq-Well (a well-array-based variation on MARS-seq)35" was incorrect. The words "and Seq-Well (a well-array-based variation on MARS-seq)35" should have been deleted. This error has been corrected in the HTML and PDF versions of the article. In addition, refs. 36–41 in the original paper have been renumbered as 35–40 and original ref. 35 has been renumbered as ref. 41 throughout the text and in Figure 1.
References
Gest, H. The discovery of microorganisms by Robert Hooke and Antoni Van Leeuwenhoek, fellows of the Royal Society. Notes Rec. R. Soc. Lond. 58, 187–201 (2004).
Arendt, D. et al. The origin and evolution of cell types. Nat. Rev. Genet. 17, 744–757 (2016).
Poulin, J.-F., Tasic, B., Hjerling-Leffler, J., Trimarchi, J.M. & Awatramani, R. Disentangling neural cell diversity using single-cell transcriptomics. Nat. Neurosci. 19, 1131–1141 (2016).
Mosmann, T.R., Cherwinski, H., Bond, M.W., Giedlin, M.A. & Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136, 2348–2357 (1986).
Orkin, S.H. Diversification of haematopoietic stem cells to specific lineages. Nat. Rev. Genet. 1, 57–64 (2000).
Zhu, J. Transcriptional regulation of Th2 cell differentiation. Immunol. Cell Biol. 88, 244–249 (2010).
Ivanov, I.I., Zhou, L. & Littman, D.R. Transcriptional regulation of Th17 cell differentiation. Semin. Immunol. 19, 409–417 (2007).
Trapnell, C. Defining cell types and states with single-cell genomics. Genome Res. 25, 1491–1498 (2015).
Kolodziejczyk, A.A., Kim, J.K., Svensson, V., Marioni, J.C. & Teichmann, S.A. The technology and biology of single-cell RNA sequencing. Mol. Cell 58, 610–620 (2015).
Eberwine, J. et al. Analysis of gene expression in single live neurons. Proc. Natl. Acad. Sci. USA 89, 3010–3014 (1992).
Lambolez, B., Audinat, E., Bochet, P., Crépel, F. & Rossier, J. AMPA receptor subunits expressed by single Purkinje cells. Neuron 9, 247–258 (1992).
Peixoto, A., Monteiro, M., Rocha, B. & Veiga-Fernandes, H. Quantification of multiple gene expression in individual cells. Genome Res. 14, 1938–1947 (2004).
Sheng, H.Z., Lin, P.X. & Nelson, P.G. Analysis of multiple heterogeneous mRNAs in single cells. Anal. Biochem. 222, 123–130 (1994).
Tietjen, I. et al. Single-cell transcriptional analysis of neuronal progenitors. Neuron 38, 161–175 (2003).
Kurimoto, K. et al. An improved single-cell cDNA amplification method for efficient high-density oligonucleotide microarray analysis. Nucleic Acids Res. 34, e42 (2006).
Kurimoto, K., Yabuta, Y., Ohinata, Y. & Saitou, M. Global single-cell cDNA amplification to provide a template for representative high-density oligonucleotide microarray analysis. Nat. Protoc. 2, 739–752 (2007).
Esumi, S. et al. Method for single-cell microarray analysis and application to gene-expression profiling of GABAergic neuron progenitors. Neurosci. Res. 60, 439–451 (2008).
Tang, F. et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 6, 377–382 (2009).
Tang, F. et al. Tracing the derivation of embryonic stem cells from the inner cell mass by single-cell RNA-Seq analysis. Cell Stem Cell 6, 468–478 (2010).
Tang, F. et al. Deterministic and stochastic allele specific gene expression in single mouse blastomeres. PLoS One 6, e21208 (2011).
Ramsköld, D. et al. Full-length mRNA-seq from single-cell levels of RNA and individual circulating tumor cells. Nat. Biotechnol. 30, 777–782 (2012).
Brouilette, S. et al. A simple and novel method for RNA-seq library preparation of single cell cDNA analysis by hyperactive Tn5 transposase. Dev. Dyn. 241, 1584–1590 (2012).
Guo, G. et al. Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev. Cell 18, 675–685 (2010).
Islam, S. et al. Characterization of the single-cell transcriptional landscape by highly multiplex RNA-seq. Genome Res. 21, 1160–1167 (2011).
Zeisel, A. et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347, 1138–1142 (2015).
Shapiro, E., Biezuner, T. & Linnarsson, S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat. Rev. Genet. 14, 618–630 (2013).
Svensson, V. et al. Power analysis of single-cell RNA-sequencing experiments. Nat. Methods 14, 381–387 (2017).
Picelli, S. et al. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat. Methods 10, 1096–1098 (2013).
Boon, W.C. et al. Increasing cDNA yields from single-cell quantities of mRNA in standard laboratory reverse transcriptase reactions using acoustic microstreaming. J. Vis. Exp. 3144, e3144 (2011).
Klein, C.A. et al. Combined transcriptome and genome analysis of single micrometastatic cells. Nat. Biotechnol. 20, 387–392 (2002).
Zhu, Y.Y., Machleder, E.M., Chenchik, A., Li, R. & Siebert, P.D. Reverse transcriptase template switching: a SMART approach for full-length cDNA library construction. Biotechniques 30, 892–897 (2001).
Baugh, L.R., Hill, A.A., Brown, E.L. & Hunter, C.P. Quantitative analysis of mRNA amplification by in vitro transcription. Nucleic Acids Res. 29, E29 (2001).
Jaitin, D.A. et al. Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types. Science 343, 776–779 (2014).
Klein, A.M. et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 161, 1187–1201 (2015).
Vickovic, S. et al. Massive and parallel expression profiling using microarrayed single-cell sequencing. Nat. Commun. 7, 13182 (2016).
Muraro, M.J. et al. A single-cell transcriptome atlas of the human pancreas. Cell Syst. 3, 385–394 (2016).
Lönnberg, T. et al. Single-cell RNA-seq and computational analysis using temporal mixture modeling resolves TH1/TFH fate bifurcation in malaria. Sci. Immunol. 2, eaal2192 (2017).
Shalek, A.K. et al. Single-cell transcriptomics reveals bimodality in expression and splicing in immune cells. Nature 498, 236–240 (2013).
Mazutis, L. et al. Single-cell analysis and sorting using droplet-based microfluidics. Nat. Protoc. 8, 870–891 (2013).
Macosko, E.Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Gierahn, T.M. et al. Seq-Well: portable, low-cost RNA sequencing of single cells at high throughput. Nat. Methods 14, 395–398 (2017).
Fan, H.C., Fu, G.K. & Fodor, S.P.A. Combinatorial labeling of single cells for gene expression cytometry. Science 347, 1258367 (2015).
Bose, S. et al. Scalable microfluidics for single-cell RNA printing and sequencing. Genome Biol. 16, 120 (2015).
Hashimshony, T., Wagner, F., Sher, N. & Yanai, I. CEL-Seq: single-cell RNA-Seq by multiplexed linear amplification. Cell Rep. 2, 666–673 (2012).
Zheng, G.X.Y. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Hochgerner, H. et al. STRT-seq-2i: dual-index 5′ single cell and nucleus RNA-seq on an addressable microwell array. Preprint at https://www.biorxiv.org/content/early/2017/04/20/126268 (2017).
Costea, P.I., Lundeberg, J. & Akan, P. TagGD: fast and accurate software for DNA Tag generation and demultiplexing. PLoS One 8, e57521 (2013).
Cusanovich, D.A. et al. Multiplex single cell profiling of chromatin accessibility by combinatorial cellular indexing. Science 348, 910–914 (2015).
Vitak, S.A. et al. Sequencing thousands of single-cell genomes with combinatorial indexing. Nat. Methods 14, 302–308 (2017).
Ramani, V. et al. Massively multiplex single-cell Hi-C. Nat. Methods 14, 263–266 (2017).
Cao, J. et al. Comprehensive single cell transcriptional profiling of a multicellular organism by combinatorial indexing. Preprint at https://www.biorxiv.org/content/early/2017/02/02/104844 (2017).
Rosenberg, A.B. et al. Scaling single cell transcriptomics through split pool barcoding. Preprint at https://www.biorxiv.org/content/early/2017/02/02/105163 (2017).
Habib, N. et al. Massively parallel single-nucleus RNA-seq with DroNc-seq. Nat. Methods 14, 955–958 (2017).
Lake, B.B. et al. Neuronal subtypes and diversity revealed by single-nucleus RNA sequencing of the human brain. Science 352, 1586–1590 (2016).
Lake, B.B. et al. A comparative strategy for single-nucleus and single-cell transcriptomes confirms accuracy in predicted cell-type expression from nuclear RNA. Sci. Rep. 7, 6031 (2017).
Lee, J.H. Quantitative approaches for investigating the spatial context of gene expression. Wiley Interdiscip. Rev. Syst. Biol. Med. 9, e1369 (2017).
Ståhl, P.L. et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 353, 78–82 (2016).
Moffitt, J.R. et al. High-performance multiplexed fluorescence in situ hybridization in culture and tissue with matrix imprinting and clearing. Proc. Natl. Acad. Sci. USA 113, 14456–14461 (2016).
Moffitt, J.R. et al. High-throughput single-cell gene-expression profiling with multiplexed error-robust fluorescence in situ hybridization. Proc. Natl. Acad. Sci. USA 113, 11046–11051 (2016).
Shah, S., Lubeck, E., Zhou, W. & Cai, L. In situ transcription profiling of single cells reveals spatial organization of cells in the mouse hippocampus. Neuron 92, 342–357 (2016).
Lee, J.H. et al. Fluorescent in situ sequencing (FISSEQ) of RNA for gene expression profiling in intact cells and tissues. Nat. Protoc. 10, 442–458 (2015).
Lee, J.H. et al. Highly multiplexed subcellular RNA sequencing in situ. Science 343, 1360–1363 (2014).
Svensson, V., Teichmann, S.A. & Stegle, O. Spatial DE: identification of spatially variable genes. Preprint at https://www.biorxiv.org/content/early/2017/11/08/143321 (2017).
Brennecke, P. et al. Accounting for technical noise in single-cell RNA-seq experiments. Nat. Methods 10, 1093–1095 (2013).
Acknowledgements
We thank Z.-S. Chong, K.N. Natarajan, T. Gomes, N. Edner and K. Meyer for reading the manuscript and giving helpful feedback. R.V.-T. is supported by an EMBO Long-Term Fellowship and a Human Frontier Science Program Long-Term Fellowship.
Author information
Authors and Affiliations
Contributions
V.S., R.V.-T. and S.A.T. wrote the manuscript. V.S. prepared Figure 1.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Sample sizes from representative scRNA-seq studies. This table details the number of single cells reported in selected scRNA-seq studies from the literature where increases in scale or particularly large data sets are mentioned. Also indicated are the protocols used and the cell isolation strategies. (XLSX 12 kb)
Rights and permissions
About this article
Cite this article
Svensson, V., Vento-Tormo, R. & Teichmann, S. Exponential scaling of single-cell RNA-seq in the past decade. Nat Protoc 13, 599–604 (2018). https://doi.org/10.1038/nprot.2017.149
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.149
This article is cited by
-
Analysis of a Single Cell RNA-seq Workflow by Random Matrix Theory Methods
Bulletin of Mathematical Biology (2025)
-
PancrESS – a meta-analysis resource for understanding cell-type specific expression in the human pancreas
BMC Genomics (2024)
-
Decoding cellular plasticity and niche regulation of limbal stem cells during corneal wound healing
Stem Cell Research & Therapy (2024)
-
deMULTIplex2: robust sample demultiplexing for scRNA-seq
Genome Biology (2024)
-
Split Pool Ligation-based Single-cell Transcriptome sequencing (SPLiT-seq) data processing pipeline comparison
BMC Genomics (2024)