Abstract
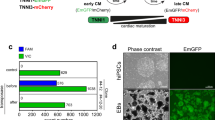
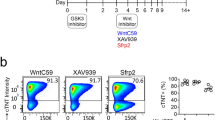
The protocol described here efficiently directs human pluripotent stem cells (hPSCs) to functional cardiomyocytes in a completely defined, growth factor– and serum-free system by temporal modulation of regulators of canonical Wnt signaling. Appropriate temporal application of a glycogen synthase kinase 3 (GSK3) inhibitor combined with the expression of β-catenin shRNA or a chemical Wnt inhibitor is sufficient to produce a high yield (0.8–1.3 million cardiomyocytes per cm2) of virtually pure (80–98%) functional cardiomyocytes in 14 d from multiple hPSC lines without cell sorting or selection. Qualitative (immunostaining) and quantitative (flow cytometry) characterization of differentiated cells is described to assess the expression of cardiac transcription factors and myofilament proteins. Flow cytometry of BrdU incorporation or Ki67 expression in conjunction with cardiac sarcomere myosin protein expression can be used to determine the proliferative capacity of hPSC-derived cardiomyocytes. Functional human cardiomyocytes differentiated via these protocols may constitute a potential cell source for heart disease modeling, drug screening and cell-based therapeutic applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kehat, I. et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Invest. 108, 407–414 (2001).
Graichen, R. et al. Enhanced cardiomyogenesis of human embryonic stem cells by a small molecular inhibitor of p38 MAPK. Differentiation 76, 357–370 (2008).
Yang, L. et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453, 524–528 (2008).
Kattman, S.J. et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 8, 228–240 (2011).
Mohr, J.C. et al. The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells. Biomaterials 31, 1885–1893 (2010).
Azarin, S.M. et al. Modulation of Wnt/β-catenin signaling in human embryonic stem cells using a 3D microwell array. Biomaterials 33, 2041–2049 (2012).
Lian, X. et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl Acad. Sci. USA 109, E1848–E1857 (2012).
Zhang, J. et al. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ. Res. 111, 1125–1136 (2012).
Laflamme, M.A. et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 25, 1015–1024 (2007).
Melkoumian, Z. et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat. Biotechnol. 28, 606–610 (2010).
Paige, S.L. et al. Endogenous Wnt/β-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS ONE 5, e11134 (2010).
Xu, X.Q. et al. Chemically defined medium supporting cardiomyocyte differentiation of human embryonic stem cells. Differentiation 76, 958–970 (2008).
Freund, C. et al. Insulin redirects differentiation from cardiogenic mesoderm and endoderm to neuroectoderm in differentiating human embryonic stem cells. Stem Cells 26, 724–733 (2008).
Hazeltine, L.B. et al. Effects of substrate mechanics on contractility of cardiomyocytes generated from human pluripotent stem cells. Int. J. Cell Biol. 2012 508294 (2012).
Ueno, S. et al. Biphasic role for Wnt/β-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl Acad. Sci. USA 104, 9685–9690 (2007).
Naito, A.T. et al. Developmental stage-specific biphasic roles of Wnt/β-catenin signaling in cardiomyogenesis and hematopoiesis. Proc. Natl Acad. Sci. USA 103, 19812–19817 (2006).
Gonzalez, R., Lee, J.W. & Schultz, P.G. Stepwise chemically induced cardiomyocyte specification of human embryonic stem cells. Angew. Chem. Int. Ed. Engl. 50, 11181–11185 (2011).
Wang, H., Hao, J. & Hong, C.C. Cardiac induction of embryonic stem cells by a small molecule inhibitor of Wnt/β-catenin signaling. ACS Chem. Biol. 6, 192–197 (2011).
Lints, T.J., Parsons, L.M., Hartley, L., Lyons, I. & Harvey, R.P. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development 119, 969 (1993).
Bu, L. et al. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature 460, 113–117 (2009).
Qyang, Y. et al. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/β-catenin pathway. Cell Stem Cell 1, 165–179 (2007).
Zhang, J. et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 104, e30–e41 (2009).
Pfannkuche, K. et al. Generation of a double-fluorescent double-selectable Cre/loxP indicator vector for monitoring of intracellular recombination events. Nat. Protoc. 3, 1510–1526 (2008).
Acknowledgements
This study was supported by US National Institutes of Health grant nos. R01 EB007534 and U01 HL099773 and National Science Foundation grant no. EFRI 0735903.
Author information
Authors and Affiliations
Contributions
X.L. designed and performed experiments, analyzed the data and wrote the paper. J.Z., S.M.A., K.Z., L.B.H., X.B. and C.H. contributed to the development of this protocol. T.J.K. and S.P.P. supervised the project, and wrote and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
T.J.K. is a founder and consultant for Cellular Dynamics International, a company that uses human stem cells for drug testing. All the other authors declare no competing financial interests.
Supplementary information
Supplementary Video 1
Spontaneous contraction of cardiomyocytes differentiated using Step 12A (GiSB method). 19-9-11 inducible β-catenin shRNA cells were treated with 12 μM CHIR99021 at day 0 and 2 μg/ml dox at 36 hr. Movie S1 shows day 15 cardiomyocytes. (WMV 4866 kb)
Supplementary Video 2
Spontaneous contraction of cardiomyocytes differentiated using Step 12A (GiSB method). 19-9-11 inducible β-catenin shRNA cells were differentiated as described in Movie S1. Movie S2 shows day 180 cardiomyocytes. (WMV 4943 kb)
Rights and permissions
About this article
Cite this article
Lian, X., Zhang, J., Azarin, S. et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc 8, 162–175 (2013). https://doi.org/10.1038/nprot.2012.150
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2012.150