Abstract
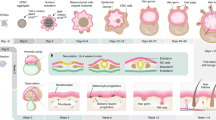
Protocols for preparing and culturing primary keratinocytes from newborn and adult mouse epidermis have evolved over the past 35 years. This protocol is now routinely applied to mice of various genetic backgrounds for in vitro studies of signaling pathways in differentiation and cell transformation, and for assessing the in vivo phenotype of altered keratinocytes in grafts of cells on immunodeficient mice. Crucial in the development and application of the procedure was the observation that keratinocytes proliferate in media of low calcium concentration, but rapidly commit to differentiation at calcium concentrations >0.07 mM after the initial attachment period. Preparing primary keratinocytes from ten newborn mice requires 2–3 h of hands-on time. Related procedures are also provided: preparing immature hair follicle buds, developing dermal hair follicles and fibroblasts from newborn mice, preparing primary keratinocytes from adult mice and grafting cell mixtures on athymic nude mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hennings, H. et al. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell 19, 245–254 (1980).
Hennings, H. Primary culture of keratinocytes from newborn mouse epidermis in medium with lowered levels of Ca2+. In Keratinocyte Methods (eds. Leigh, I. & Watt, F.M.) 21–23 (Cambridge University Press, Cambridge, UK, 1994).
Yuspa, S.H., Kilkenny, A.E., Steinert, P.M. & Roop, D.R. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J. Cell Biol. 109, 1207–1217 (1989).
Dlugosz, A.A., Glick, A.B., Tennenbaum, T., Weinberg, W.C. & Yuspa, S.H. Isolation and utilization of epidermal keratinocytes for oncogene research. In Methods in Enzymology Vol. 254, 3–20 (Academic Press, New York, 1995).
Kulesz-Martin, M.F., Koehler, B., Hennings, H. & Yuspa, S.H. Quantitative assay for carcinogen altered differentiation in mouse epidermal cells. Carcinogenesis 1, 995–1006 (1980).
Kilkenny, A.E., Morgan, D., Spangler, E.F. & Yuspa, S.H. Correlation of initiating potency of skin carcinogens with potency to induce resistance to terminal differentiation in cultured mouse keratinocytes. Cancer Res. 45, 2219–2225 (1985).
Kawamura, H., Strickland, J.E. & Yuspa, S.H. Association of resistance to terminal differentiation with initiation of carcinogenesis in adult mouse epidermal cells. Cancer Res. 45, 2748–2752 (1985).
Forslind, B. Particle probe analysis in the study of skin physiology. Scan. Electron Microsc. 3, 1007–1014 (1986).
Elias, P.M. et al. Formation of the epidermal calcium gradient coincides with key milestones of barrier ontogenesis in the rodent. J. Invest. Dermatol. 110, 399–404 (1998).
Fusenig, N.E. & Worst, P.K. Mouse epidermal cell cultures. II. Isolation, characterization and cultivation of epidermal cells from perinatal mouse skin. Exp. Cell Res. 93, 443–457 (1975).
Morris, R.J., Haynes, A.C., Fischer, S.M. & Slaga, T.J. Concomitant proliferation and formation of a stratified epithelial sheet by explant outgrowth of epidermal keratinocytes from adult mice. In Vitro Cell. Dev. Biol. 27A, 886–895 (1991).
Hager, B., Bickenbach, J.R. & Fleckman, P. Long-term culture of murine epidermal keratinocytes. J. Invest. Dermatol. 112, 971–976 (1999).
Yuspa, S.H., Koehler, B., Kulesz-Martin, M. & Hennings, H. Clonal growth of mouse epidermal cells in medium with reduced calcium concentration. J. Invest. Dermatol. 76, 144–146 (1981).
Kaighn, M.E., Camalier, R.F., Bertolero, F. & Saffiotti, U. Spontaneous establishment and characterization of mouse keratinocyte cell lines in serum-free medium. In Vitro Cell. Dev. Biol. 24, 845–854 (1988).
Weissman, B.E. & Aaronson, S.A. BALB and Kirsten murine sarcoma viruses alter growth and differentiation of EGF-dependent balb/c mouse epidermal keratinocyte lines. Cell 32, 599–606 (1983).
Backendorf, C. et al. Repair characteristics and differentiation propensity of long term cultures of epidermal keratinocytes derived from normal and NER-deficient mice. DNA Repair (Amst.) 4, 1325–1336 (2005).
Castilho, R.M. et al. Requirement of Rac1 distinguishes follicular from interfollicular epithelial stem cells. Oncogene 26, 5078–5085 (2007).
Weinberg, W.C. et al. Reconstitution of hair follicle development in vivo: determination of follicle formation, hair growth and hair quality by dermal cells. J. Invest. Dermatol. 100, 229–236 (1993).
Yuspa, S.H. et al. Regulation of hair follicle development: an in vitro model for hair follicle invasion of dermis and associated connective tissue remodeling. J. Invest. Dermatol. 101 (1 suppl.): 27S–32S (1993).
Scandurro, A.B. et al. Immortalized rat whisker dermal papilla cells cooperate with mouse immature hair follicle buds to activate type IV procollagenases in collagen matrix coculture: correlation with ability to promote hair follicle development in nude mouse grafts. J. Invest. Dermatol. 105, 177–183 (1995).
Roop, D.R. et al. An activated Harvey ras oncogene produces benign tumours on mouse epidermal tissue. Nature 323, 822–824 (1986).
Hansen, L.A. et al. The epidermal growth factor receptor is required to maintain the proliferative population in the basal compartment of epidermal tumors. Cancer Res. 60, 3328–3332 (2000).
Acknowledgements
Many current and former members of the laboratory have been instrumental in refining the techniques described. Several of them are acknowledged by citing their publications in the text. Special thanks are owed to Christophe Cataisson, a current member of the laboratory, for contributing the images comprising Figure 4.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lichti, U., Anders, J. & Yuspa, S. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc 3, 799–810 (2008). https://doi.org/10.1038/nprot.2008.50
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2008.50