Abstract
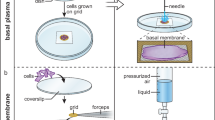
In this protocol, we describe cryoimmunolabeling methods for the subcellular localization of proteins and certain lipids. The methods start with chemical fixation of cells and tissue in formaldehyde (FA) and/or glutaraldehyde (GA), sometimes supplemented with acrolein. Cell and tissue blocks are then immersed in 2.3 M sucrose before freezing in liquid nitrogen. Thin cryosections, cut in an ultracryotome, can be single- or multiple immunolabeled with differently sized gold particles, contrasted and viewed in an electron microscope. Semi-thin cryosections can be used for immunofluorescence microscopy. We describe the detailed procedures that have been developed and tested in practice in our laboratory during the past decades.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
van Donselaar, E., Posthuma, G., Zeuschner, D., Humbel, B.M. & Slot, J.W. Immunogold labeling of cryosections from high-pressure frozen cells. Traffic 8, 471–485 (2007).
Roth, J., Bendayan, M. & Orci, L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J. Histochem. Cytochem. 26, 1074–1081 (1978).
Geuze, H.J., Slot, J.W., van der Ley, P.A. & Scheffer, R.C. Use of colloidal gold particles in double-labeling immunoelectron microscopy of ultrathin frozen tissue sections. J. Cell Biol. 89, 653–665 (1981).
Slot, J.W. & Geuze, H.J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur. J. Cell Biol. 38, 87–93 (1985).
Slot, J.W. & Geuze, H.J. Sizing of protein A-colloidal gold probes for immunoelectron microscopy. J. Cell Biol. 90, 533–536 (1981).
Tokuyasu, K.T. Immunocytochemistry of ultrathin frozen sections. Histochem. J. 12, 381–403 (1980).
Tokuyasu, K.T. A technique for ultracryotomy of cell suspensions and tissues. J. Cell Biol. 57, 551–565 (1973).
Tokuyasu, K.T. Membranes as observed in frozen sections. J. Ultrastruct. Res. 55, 281–287 (1976).
Tokuyasu, K.T. Use of poly(vinylpyrrolidone) and poly(vinyl alcohol) for cryoultramicrotomy. Histochem. J. 21, 163–171 (1989).
Liou, W., Geuze, H.J., Geelen, M.J. & Slot, J.W. The autophagic and endocytic pathways converge at the nascent autophagic vacuoles. J. Cell Biol. 136, 61–70 (1997).
Griffiths, G. Fixation for structure preservation and immunocytochemistry. in Fine Structure Immuno-cytochemistry Chapter 3 26–89 (Springer-Verlag Berlin Heidelberg, Germany, 1993).
Tokuyasu, K. & Okamura, S. A new method for making glass knives for thin sectioning. J. Biophys. Biochem. Cytol. 6, 305–308 (1959).
Johnson, T.J.A. Aldehyde fixatives: quantification of acid-producing reactions. J. Electron Microsc. 2, 129–138 (1985).
Liou, W., Geuze, H.J. & Slot, J.W. Improving structural integrity of cryosections for immunogold labeling. Histochem. Cell Biol. 106, 41–58 (1996).
Oorschot, V., de Wit, H., Annaert, W.G. & Klumperman, J. A novel flat-embedding method to prepare ultrathin cryosections from cultured cells in their in situ orientation. J. Histochem. Cytochem. 50, 1067–1080 (2002).
Griffiths, G., McDowall, A., Back, R. & Dubochet, J. On the preparation of cryosections for immunocytochemistry. J. Ultrastruct. Res. 89, 65–78 (1984).
Geuze, H.J. & Slot, J.W. Disproportional immunostaining patterns of two secretory proteins in guinea pig and rat exocrine pancreas cells. An immunoferritin and fluorescence study. Eur. J. Cell Biol. 21, 93–100 (1980).
Griffith, J.M. & Posthuma, G. A reliable and convenient method to store ultrathin thawed cryosections prior to immunolabeling. J. Histochem. Cytochem. 50, 57–62 (2002).
Slot, J.W., Posthuma, G., Chang, L.Y., Crapo, J.D. & Geuze, H.J. Quantitative aspects of immunogold labeling in embedded and in nonembedded sections. Am. J. Anat. 185, 271–281 (1989).
Zeuschner, D. et al. Immuno-electron tomography of ER exit sites reveals the existence of free COPII-coated transport carriers. Nat. Cell Biol. 8, 377–383 (2006).
Herpers, B. & Rabouille, C. mRNA localization and ER-based protein sorting mechanisms dictate the use of transitional endoplasmic reticulum-golgi units involved in gurken transport in Drosophila oocytes. Mol. Biol. Cell 15, 5306–5317 (2004).
Möbius, W. et al. Immunoelectron microscopic localization of cholesterol using biotinylated and non-cytolytic perfringolysin O. J. Histochem. Cytochem. 50, 43–55 (2002).
Möbius, W. et al. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic 4, 222–231 (2003).
Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspension. Nature (Phys. Sci.) 241, 20–22 (1973).
Zsigmondy, R. & Thiessen, P.A. Das Kolloidale Gold (Akademische Verlagsgesellschaft M.B.H., Leipzig, Germany, 1925).
Horisberger, M. & Rosset, J. Colloidal gold, a useful marker for transmission and scanning electron microscopy. J. Histochem. Cytochem. 25, 295–305 (1977).
De Roe, C., Courtoy, P.J. & Baudhuin, P. A model of protein-colloidal gold interactions. J. Histochem. Cytochem. 35, 1191–1198 (1987).
Horisberger, M. & Clerc, M.F. Labelling of colloidal gold with protein A. A quantitative study. Histochemistry 82, 219–223 (1985).
Acknowledgements
We are very grateful for the long and excellent collaboration with Elly van Donselaar, Janice Griffith, Viola Oorschot and George Posthuma. Their technical skills and devotion to deliver optimal results were essential to set the standards of the above protocols. From outside, our group we like to acknowledge Dr. Gareth Griffiths with whom we had fruitful discussions all over the years. In particular, we thank Dr. Kyoteru Tokuyasu, our master.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Slot, J., Geuze, H. Cryosectioning and immunolabeling. Nat Protoc 2, 2480–2491 (2007). https://doi.org/10.1038/nprot.2007.365
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.365