Abstract
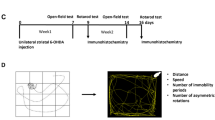
This protocol describes our method of producing a reliable mouse model of Parkinson's disease (PD) using the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). We discuss the particulars of the model, provide key references and outline what investigators need to know to develop the MPTP mouse model of PD safely and successfully. Completion of this protocol depends on the regimen of MPTP used and on the actual planned studies, which often range from 7 to 30 d. This protocol calls for implementation of safety measures and for the acquisition of several pieces of equipment, which are a one-time investment worth making if one elects to use this model on a regular basis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fahn, S., Przedborski, S. Parkinsonism, In Merritt's Neurology (ed. Rowland, L.P.) 828–846 (Lippincott Williams & Wilkins, New York, 2005).
Dauer, W. & Przedborski, S. Parkinson's disease: mechanisms and models. Neuron 39, 889–909 (2003).
Przedborski, S. & Tieu, K. Toxic animal models. In Neurodegenerative diseases: Neurobiology, pathogenesis and therapeutics (eds. Beal, M.F., Lang, A.E. & Ludolph, A.) 196–221 (Cambridge, New York, 2005).
Langston, J.W., Ballard, P. & Irwin, I. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219, 979–980 (1983).
Sotelo, C., Javoy, F., Agid, Y. & Glowinski, J. Injection of 6-hydroxydopamine in the substantia nigra of the rat. I. Morphological study. Brain Res. 58, 269–290 (1973).
Betarbet, R. et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat. Neurosci. 3, 1301–1306 (2000).
Bove, J. et al. Proteasome inhibition and Parkinson's disease modeling. Ann. Neurol. 60, 260–264 (2006).
Przedborski, S. et al. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J. Neurochem. 76, 1265–1274 (2001).
Sonsalla, P.K. & Heikkila, R.E. The influence of dose and dosing interval on MPTP-induced dopaminergic neurotoxicity in mice. Eur. J. Pharmacol. 129, 339–345 (1986).
Fornai, F. et al. Parkinson-like syndrome induced by continuous MPTP infusion: Convergent roles of the ubiquitin-proteasome system and \{alpha}-synuclein. Proc. Natl. Acad. Sci. USA 102, 3413–3418 (2005).
Meredith, G.E. et al. Lysosomal malfunction accompanies alpha-synuclein aggregation in a progressive mouse model of Parkinson's disease. Brain Res. 956, 156–165 (2002).
Heikkila, R.E., Sieber, B.A., Manzino, L. & Sonsalla, P.K. Some features of the nigrostriatal dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the mouse. Mol. Chemic. Neuropathol. 10, 171–183 (1989).
Giovanni, A., Sieber, B.A., Heikkila, R.E. & Sonsalla, P.K. Correlation between the neostriatal content of the 1-methyl-4-phenylpyridinium species and dopaminergic neurotoxicity following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration to several strains of mice. J. Pharmacol. Exp. Ther. 257, 691–697 (1991).
Giovanni, A., Sieber, B.-A., Heikkila, R.E. & Sonsalla, P.K. Studies on species sensitivity to the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Part 1: Systemic administration. J. Pharmacol. Exp. Ther. 270, 1000–1007 (1994).
Giovanni, A., Sonsalla, P.K. & Heikkila, R.E. Studies on species sensitivity to the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Part 2: Central administration of 1-methyl-4-phenylpyridinium. J. Pharmacol. Exp. Ther. 270, 1008–1014 (1994).
Miller, D.B., Ali, S.F., O'Callaghan, J.P. & Laws, S.C. The impact of gender and estrogen on striatal dopaminergic neurotoxicity. Ann. N. Y. Acad. Sci. 844, 153–165 (1998).
Staal, R.G. & Sonsalla, P.K. Inhibition of brain vesicular monoamine transporter (VMAT2) enhances 1-methyl-4-phenylpyridinium neurotoxicity in vivo in rat striata. J. Pharmacol. Exp. Ther. 293, 336–342 (2000).
Javitch, J.A., D'Amato, R.J., Strittmatter, S.M. & Snyder, S.H. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridinium by dopamine neurons explain selective toxicity. Proc. Natl. Acad. Sci. USA 82, 2173–2177 (1985).
Liu, Y., Roghani, A. & Edwards, R.H. Gene transfer of a reserpine-sensitive mechanism of resistance to N-methyl-4-phenylpyridinium. Proc. Natl. Acad. Sci. USA 89, 9074–9078 (1992).
Ramsay, R.R. & Singer, T.P. Energy-dependent uptake of N-methyl-4-phenylpyridinium, the neurotoxic metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, by mitochondria. J. Biol. Chem. 261, 7585–7587 (1986).
Jackson-Lewis, V., Jakowec, M., Burke, R.E. & Przedborski, S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration 4, 257–269 (1995).
Tatton, N.A. & Kish, S.J. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 77, 1037–1048 (1997).
Liberatore, G. et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 5, 1403–1409 (1999).
Przedborski, S. et al. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J. Neurosci. 12, 1658–1667 (1992).
Youdim, M.B.H. Assay and purification of brain monoamine oxidase, in Eds. Marks, N. & Rodnight, R. Research Methods in Neurochemistry, Vol. 3, 167–208 (Plenum Press, New York, 1975).
Tieu, K. et al. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Invest. 112, 892–901 (2003).
Chiba, K., Kubota, E., Miyakawa, T., Kato, Y. & Ishizaki, T. Characterization of hepatic microsomal metabolism as an in vivo detoxication pathway of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. J. Pharmacol. Exp. Ther. 246, 1108–1115 (1988).
Yang, S.C., Markey, S.P., Bankiewicz, K.S., London, W.T. & Lunn, G. Recommended safe practices for using the neurotoxin MPTP in animal experiments. Lab. Anim. Sci. 38, 563–567 (1988).
Johannessen, J.N., Chiueh, C.C., Herkenham, M. & Markey, C.J. Relationship of the in vivo metabolism of MPTP to toxicity. In MPTP: A Neurotoxin Producing a Parkinsonian Syndrome (eds. Markey, S.P., Castagnoli, N. Jr., Trevor, A.J. & Kopin, I.J.) 173–189 (Academic Press, Orlando, 1986).
Markey, S.P., Johannessen, J.N., Chiueh, C.C., Burns, R.S. & Herkenham, M.A. Intraneuronal generation of a pyridinium metabolite may cause drug-induced parkinsonism. Nature 311, 464–467 (1984).
Kiernan, J.A. Histological and Histochemical Methods Theory and Practice (Butterworth Heinemann, Oxford, 1999).
Vila, M. et al. Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Proc. Natl. Acad. Sci. USA 98, 2837–2842 (2001).
Shinka, T., Castagnoli, N., Jr., Wu, E.Y., Hoag, M.K. & Trevor, A.J. Cation-exchange high-performance liquid chromatography assay for the nigrostriatal toxicant 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and its monoamine oxidase B generated metabolites in brain tissues. J. Chromatogr. 398, 279–287 (1987).
Acknowledgements
The authors wish to thank Drs. Cristina Malagelada Grau, Delphine Prou, Du-Chu Wu, Diane Re and Chun Zhou for their insightful comments on this manuscript. The authors wish to acknowledge the support of the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke Grants R01 NS42269, P50 NS38370 and P01 NS11766; NIH/National Institute of Aging Grant AG R01 21617; National Institute of Environmental Health Sciences Grant ES013177; US Department of Defense Grant DAMD 17-03-1; the Parkinson's Disease Foundation Grant CU51523606, and the Muscular Dystrophy Association/Wings-over-Wall Street.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Data
MPTP Core Experiment Log (PDF 15 kb)
Supplementary Information
MPTP Core Experiment Log (PDF 19 kb)
Supplementary Video
Mouse brain dissection tutorial (MOV 61856 kb)
Rights and permissions
About this article
Cite this article
Jackson-Lewis, V., Przedborski, S. Protocol for the MPTP mouse model of Parkinson's disease. Nat Protoc 2, 141–151 (2007). https://doi.org/10.1038/nprot.2006.342
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.342
This article is cited by
-
MicroRNA-218-5p-Ddx41 axis restrains microglia-mediated neuroinflammation through downregulating type I interferon response in a mouse model of Parkinson’s disease
Journal of Translational Medicine (2024)
-
Long-term benefits of hematopoietic stem cell-based macrophage/microglia delivery of GDNF to the CNS in a mouse model of Parkinson’s disease
Gene Therapy (2024)
-
Non-Faradaic optoelectrodes for safe electrical neuromodulation
Nature Communications (2024)
-
Beneficial role of capsaicin through modulation of mitochondrial functions in MPTP-injected mice
Neuroscience and Behavioral Physiology (2024)
-
Corydaline alleviates Parkinson’s disease by regulating autophagy and GSK-3β phosphorylation
Psychopharmacology (2024)