Abstract
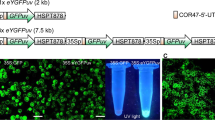
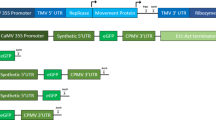
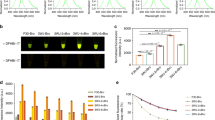
Expression and tracking of fluorescent fusion proteins has revolutionized our understanding of basic concepts in cell biology. The protocol presented here has underpinned much of the in vivo results highlighting the dynamic nature of the plant secretory pathway. Transient transformation of tobacco leaf epidermal cells is a relatively fast technique to assess expression of genes of interest. These cells can be used to generate stable plant lines using a more time-consuming, cell culture technique. Transient expression takes from 2 to 4 days whereas stable lines are generated after approximately 2 to 4 months.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lippincott-Schwartz, J. & Patterson, G.H. Development and use of fluorescent protein markers in living cells. Science 300, 87–91 (2003).
Chapman, S., Oparka, K.J. & Roberts, A.G. New tools for in vivo fluorescence tagging. Curr. Opin. Plant Biol. 8, 565–573 (2005).
Lukyanov, K.A., Chudakov, D.M., Lukyanov, S. & Verkhusha, V.V. Photoactivatable fluorescent proteins. Nat. Rev. Mol. Cell Biol. 6, 885–890 (2005).
Runions, J., Hawes, C. & Kurup, S. Fluorescent protein fusions for protein localization in plants. in Methods in Molecular Biology: Protein Targeting Protocols (ed. van der Giezen, M.) Humana Press, New Jersey, USA, in the press.
Runions, J., Brach, T., Kühner, S. & Hawes, C. Photoactivation of GFP reveals protein dynamics within the endoplasmic reticulum. J. Exp. Bot. 57, 43–50 (2006).
Hawes, C. Cell biology of the plant Golgi apparatus. New Phytol. 165, 29–44 (2005).
Brandizzi, F., Irons, S.L., Johansen, J., Kotzer, A. & Neumann, U. GFP is the way to glow: bioimaging of the plant endomembrane system. J. Microsc. 214, 138–158 (2004).
Kotzer, A.M. et al. AtRabF2b (Ara7) acts on the vacuolar trafficking pathway in tobacco leaf epidermal cells. J. Cell Sci. 117, 6377–6389 (2004).
Sparkes, I.A., Hawes, C. & Baker, A. AtPEX2 and AtPEX10 are targeted to peroxisomes independently of known endoplasmic reticulum trafficking routes. Plant Physiol. 139, 690–700 (2005).
Irons, S.L., Evans, D.E. & Brandizzi, F. The first 238 amino acids of the human lamin B receptor are targeted to the nuclear envelop in plants. J. Exp. Bot. 54, 943–950 (2003).
Walter, M. et al. Visualisation of protein interactions in living plants cells using bimolecular fluorescence complementation. Plant J. 40, 428–438.
daSilva, L.L.P. et al. ER export sites and Golgi bodies behave as single mobile secretory units in plant cells. Plant Cell 16, 1753–1771 (2004).
Clough, S.J. & Bent, A.F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . Plant J. 16, 735–743 (1998).
Zhang, X., Henriques, R., Lin, S.-S., Niu, Q.-W. & Chua, N.-H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protocols 1, 641–646 (2006).
Valvekens, D., van Montagu, M. & van Lijsebettens, M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc. Natl. Acad. Sci. USA 85, 5536–5540 (1988).
Sparkes, I.A. et al. An Arabidopsis pex10 null mutant is embryo lethal, implicating peroxisomes in an essential role during plant embryogenesis. Plant Physiol. 133, 1809–1819 (2003).
Earley, K.W. et al. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 45, 616–629 (2006).
Hellen, R., Mullineaux, P. & Klee, H. Technical focus: a guide to Agrobacterium binary Ti vectors. Trends Plant Sci. 5, 446–451 (2000).
Wroblewski, T., Tomczak, A. & Michelmore, R. Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis . Plant Biotechnol. J. 3, 259–273 (2005).
Hofgen, R. & Willmitzer, L. Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res. 16, 9877 (1988).
Evans, D.E., Coleman, J.O.D. & Kearns, A. Plant Cell culture. BIOS Scientific Publishers, London, (2003).
Boevink, P. et al. Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant J. 15, 441–447 (1998).
Acknowledgements
We are grateful to members of C. Hawes' and I. Moore's laboratories for fruitful discussions and members of the laboratories who have helped refine these techniques over the years. The work reported here was funded by various BBSRC grants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sparkes, I., Runions, J., Kearns, A. et al. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat Protoc 1, 2019–2025 (2006). https://doi.org/10.1038/nprot.2006.286
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.286
This article is cited by
-
Capture of regulatory factors via CRISPR–dCas9 for mechanistic analysis of fine-tuned SERRATE expression in Arabidopsis
Nature Plants (2024)
-
PbrCSP1, a pollen tube–specific cold shock domain protein, is essential for the growth and cold resistance of pear pollen tubes
Molecular Breeding (2024)
-
Establishment of an efficient callus transient transformation system for Vitis vinifera cv. ‘Chardonnay’
Protoplasma (2024)
-
GhHB14_D10 and GhREV_D5, two HD-ZIP III transcription factors, play a regulatory role in cotton fiber secondary cell wall biosynthesis
Plant Cell Reports (2024)
-
The evolution and functional divergence of FT-related genes in controlling flowering time in Brassica rapa ssp. rapa
Plant Cell Reports (2024)