Abstract
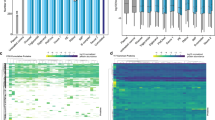
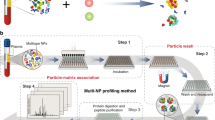
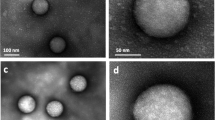
In biological fluids, proteins bind to the surface of nanoparticles to form a coating known as the protein corona, which can critically affect the interaction of the nanoparticles with living systems. As physiological systems are highly dynamic, it is important to obtain a time-resolved knowledge of protein-corona formation, development and biological relevancy. Here we show that label-free snapshot proteomics can be used to obtain quantitative time-resolved profiles of human plasma coronas formed on silica and polystyrene nanoparticles of various size and surface functionalization. Complex time- and nanoparticle-specific coronas, which comprise almost 300 different proteins, were found to form rapidly (<0.5 minutes) and, over time, to change significantly in terms of the amount of bound protein, but not in composition. Rapid corona formation is found to affect haemolysis, thrombocyte activation, nanoparticle uptake and endothelial cell death at an early exposure time.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Riehemann, K. et al. Nanomedicine—challenge and perspectives. Angew. Chem. Int. Ed. 48, 872–897 (2009).
Chauhan, V. P. et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nature Nanotech. 7, 383–388 (2012).
von Maltzahn, G. et al. Nanoparticles that communicate in vivo to amplify tumour targeting. Nature Mater. 10, 545–552 (2012).
Minchin, R. Nanomedicine: sizing up targets with nanoparticles. Nature Nanotech. 3, 12–13 (2008).
Teli, M. K., Mutalik, S. & Rajanikant, G. K. Nanotechnology and nanomedicine: going small means aiming big. Curr. Pharm. Des. 16, 1882–1892 (2010).
Smita, S. et al. Nanoparticles in the environment: assessment using the causal diagram approach. Environ. Health 11 (suppl. 1), S13 (2012).
Monopoli, M. P., Aberg, C., Salvati, A. & Dawson, K. A. Biomolecular coronas provide the biological identity of nanosized materials. Nature Nanotech. 7, 779–786 (2012).
Nel, A. E. et al. Understanding biophysicochemical interactions at the nano–bio interface. Nature Mater. 8, 543–557 (2009).
Nystrom, A. M. & Fadeel, B. Safety assessment of nanomaterials: implications for nanomedicine. J. Control. Release 161, 403–408 (2012).
Thomas, C. R. et al. Nanomaterials in the environment: from materials to high-throughput screening to organisms. ACS Nano 5, 13–20 (2011).
Oberdörster, G. Safety assessment for nanotechnology and nanomedicine: concepts of nanotoxicology. J. Intern. Med. 267, 89–105 (2011).
Xia, X. R., Monteiro-Riviere, N. A. & Riviere, J. E. An index for characterization of nanomaterials in biological systems. Nature Nanotech. 5, 671–675 (2010).
Monopoli, M. P., Bombelli, F. B. & Dawson, K. A. Nanobiotechnology: nanoparticle coronas take shape. Nature Nanotech. 6, 11–12 (2011).
Monopoli, M. P. et al. Physical-chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 133, 2525–2534 (2011).
Tenzer, S. et al. Nanoparticle size is a critical physicochemical determinant of the human blood plasma corona: a comprehensive quantitative proteomic analysis. ACS Nano 5, 7155–7167 (2011).
Zhang, H. et al. Quantitative proteomics analysis of adsorbed plasma proteins classifies nanoparticles with different surface properties and size. Proteomics 11, 4569–4577 (2011).
Dobrovolskaia, M. A., Germolec, D. R. & Weaver, J. L. Evaluation of nanoparticle immunotoxicity. Nature Nanotech. 4, 411–414 (2009).
Salvati, A. et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nature Nanotech. 8, 137–143 (2013).
Casals, E., Pfaller, T., Duschl, A., Oostingh, G. J. & Puntes, V. Time evolution of the nanoparticle protein corona. ACS Nano 4, 3623–3632 (2010).
Jaskiewicz, K. et al. Probing bioinspired transport of nanoparticles into polymersomes. Angew. Chem. Int. Ed. 51, 4613–4617 (2012).
Lunov, O. et al. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano 5, 1657–1669 (2011).
Lesniak, A. et al. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano 6, 5845–5857 (2012).
Van Hoecke, K. et al. Ecotoxicity and uptake of polymer coated gold nanoparticles. Nanotoxicology 7, 37–47 (2013).
Cedervall, T. et al. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl Acad. Sci. USA 104, 2050–2055 (2007).
Walczyk, D., Bombelli, F. B., Monopoli, M. P., Lynch, I. & Dawson, K. A. What the cell ‘sees’ in bionanoscience. J. Am. Chem. Soc. 132, 5761–5768 (2010).
Lundqvist, M. et al. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl Acad. Sci. USA 105, 14265–14270 (2008).
Huhn, D. et al. Polymer-coated nanoparticles interacting with proteins and cells: focusing on the sign of the net charge. ACS Nano 7, 3253–3263 (2013).
Vroman, L. Effect of absorbed proteins on the wettability of hydrophilic and hydrophobic solids. Nature 196, 476–477 (1962).
Lundqvist, M. et al. The evolution of the protein corona around nanoparticles: a test study. ACS Nano 5, 7503–7509 (2011).
Gebauer, J. S. et al. Impact of the nanoparticle–protein corona on colloidal stability and protein structure. Langmuir 28, 9673–9679 (2012).
Dell'Orco, D., Lundqvist, M., Oslakovic, C., Cedervall, T. & Linse, S. Modeling the time evolution of the nanoparticle–protein corona in a body fluid. PLoS One 5, e10949 (2010).
Calzolai, L., Franchini, F., Gilliland, D. & Rossi, F. Protein–nanoparticle interaction: identification of the ubiquitin–gold nanoparticle interaction site. Nano Lett. 10, 3101–3105 (2010).
Vogler, E. A. Protein adsorption in three dimensions. Biomaterials 33, 1201–1237 (2012).
Hamad, I. et al. Distinct polymer architecture mediates switching of complement activation pathways at the nanosphere–serum interface: implications for stealth nanoparticle engineering. ACS Nano 4, 6629–6638 (2010).
Lunov, O. et al. Lysosomal degradation of the carboxydextran shell of coated superparamagnetic iron oxide nanoparticles and the fate of professional phagocytes. Biomaterials 31, 9015–9022 (2010).
Sim, R. B. & Wallis, R. Surface properties: immune attack on nanoparticles. Nature Nanotech. 6, 80–81 (2011).
Rivera Gil, P., Oberdörster, G., Elder, A., Puntes, V. & Parak, W. J. Correlating physico-chemical with toxicological properties of nanoparticles: the present and the future. ACS Nano 4, 5527–5531 (2011).
Schousboe, I. & Nystrom, B. High molecular weight kininogen binds to laminin—characterization and kinetic analysis. FEBS J. 276, 5228–5238 (2009).
Zensi, A. et al. Human serum albumin nanoparticles modified with apolipoprotein A-I cross the blood–brain barrier and enter the rodent brain. J. Drug Target. 18, 842–848 (2010).
Zensi, A. et al. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones. J. Control. Release 137, 78–86 (2009).
Aggarwal, P., Hall, J. B., McLeland, C. B., Dobrovolskaia, M. A. & McNeil, S. E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 61, 428–437 (2009).
Reinhardt, C. et al. Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature 483, 627–631 (2011).
Engels, K. et al. NO signaling confers cytoprotectivity through the survivin network in ovarian carcinomas. Cancer Res. 68, 5159–5166 (2008).
Knauer, S. K. et al. Functional characterization of novel mutations affecting survivin (BIRC5)-mediated therapy resistance in head and neck cancer patients. Hum. Mutat. 34, 395–404 (2012).
Bier, C. et al. Allosteric inhibition of Taspase1's pathobiological activity by enforced dimerization in vivo. FASEB J. 26, 3421–3429 (2012).
Bauer, M. et al. Poly(2-ethyl-2-oxazoline) as alternative for the stealth polymer poly(ethylene glycol): comparison of in vitro cytotoxicity and hemocompatibility. Macromol. Biosci. 12, 986–998 (2012).
Tenzer, S. et al. Proteome-wide characterization of the RNA-binding protein RALY-interactome using the in vivo-biotinylation-pulldown-quant (iBioPQ) approach. J. Proteome Res. 12, 2869–2884 (2013).
Acknowledgements
This work was supported by Grants DFG-SPP1313 and DFG-SFB490/Z3 and by BMBF-NanoKon/NanoMed/MRCyte, Zeiss-ChemBioMed, University Mainz Forschungszentrum Immunologie, Research Center for Immunology and Stiftung Rheinland-Pfalz (NANOSCH, NanoScreen). We thank R. Spohrer for preparation of the manuscript sample, S. Schneider for technical assistance and R. Zellner for discussions.
Author information
Authors and Affiliations
Contributions
R.H.S., S.T. and D.D. conceived and designed the study. S.T., D.D., J.K., V.F., R.H., F.S., K.K. and S.K.K. performed the experiments. A.M., F.S., D.F., K.L. and M.M. synthesized and characterized nanoparticles. S.T., J.K., D.F., C.R., K.L., H.S., M.M., S.K.K. and R.H.S. analysed data. R.H.S., S.K.K. and S.T. wrote the manuscript and Supplementary Information.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 30771 kb)
Supplementary information
Supplementary Table 3 (XLSX 191 kb)
Supplementary information
Supplementary Table 4 (XLSX 501 kb)
Supplementary information
Supplementary Table 6 (XLSX 60 kb)
Supplementary information
Supplementary Table 7 (XLSX 258 kb)
Rights and permissions
About this article
Cite this article
Tenzer, S., Docter, D., Kuharev, J. et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nature Nanotech 8, 772–781 (2013). https://doi.org/10.1038/nnano.2013.181
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2013.181
This article is cited by
-
Targeting strategies with lipid vectors for nucleic acid supplementation therapy in Fabry disease: a systematic review
Drug Delivery and Translational Research (2024)
-
Engineering tumoral vascular leakiness with gold nanoparticles
Nature Communications (2023)
-
Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs
Nature Reviews Materials (2023)
-
Affinity-based and in a single step purification of recombinant horseradish peroxidase A2A isoenzyme produced by Pichia pastoris
Bioprocess and Biosystems Engineering (2023)
-
To Waste or Not to Waste: Questioning Potential Health Risks of Micro- and Nanoplastics with a Focus on Their Ingestion and Potential Carcinogenicity
Exposure and Health (2023)