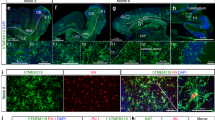
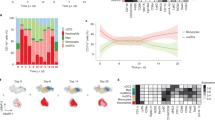
Abstract
Microglia are crucially important myeloid cells in the CNS and constitute the first immunological barrier against pathogens and environmental insults. The factors controlling microglia recruitment from the blood remain elusive and the direct circulating microglia precursor has not yet been identified in vivo. Using a panel of bone marrow chimeric and adoptive transfer experiments, we found that circulating Ly-6ChiCCR2+ monocytes were preferentially recruited to the lesioned brain and differentiated into microglia. Notably, microglia engraftment in CNS pathologies, which are not associated with overt blood-brain barrier disruption, required previous conditioning of brain (for example, by direct tissue irradiation). Our results identify Ly-6ChiCCR2+ monocytes as direct precursors of microglia in the adult brain and establish the importance of local factors in the adult CNS for microglia engraftment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hanisch, U.K. Microglia as a source and target of cytokines. Glia 40, 140–155 (2002).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005).
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758 (2005).
Streit, W.J., Walter, S.A. & Pennell, N.A. Reactive microgliosis. Prog. Neurobiol. 57, 563–581 (1999).
Simard, A.R., Soulet, D., Gowing, G., Julien, J.P. & Rivest, S. Bone marrow–derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron 49, 489–502 (2006).
Hickey, W.F. & Kimura, H. Perivascular microglial cells of the CNS are bone marrow–derived and present antigen in vivo. Science 239, 290–292 (1988).
Hickey, W.F., Vass, K. & Lassmann, H. Bone marrow–derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. J. Neuropathol. Exp. Neurol. 51, 246–256 (1992).
Priller, J. et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat. Med. 7, 1356–1361 (2001).
Wehner, T. et al. Bone marrow-derived cells expressing green fluorescent protein under the control of the glial fibrillary acidic protein promoter do not differentiate into astrocytes in vitro and in vivo. J. Neurosci. 23, 5004–5011 (2003).
Williams, A.E., Lawson, L.J., Perry, V.H. & Fraser, H. Characterization of the microglial response in murine scrapie. Neuropathol. Appl. Neurobiol. 20, 47–55 (1994).
Priller, J. et al. Early and rapid engraftment of bone marrow–derived microglia in scrapie. J. Neurosci. 26, 11753–11762 (2006).
Djukic, M. et al. Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. Brain 129, 2394–2403 (2006).
Heppner, F.L. et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat. Med. 11, 146–152 (2005).
Kokovay, E. & Cunningham, L.A. Bone marrow–derived microglia contribute to the neuroinflammatory response and express iNOS in the MPTP mouse model of Parkinson's disease. Neurobiol. Dis. 19, 471–478 (2005).
Malm, T.M. et al. Bone marrow–derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol. Dis. 18, 134–142 (2005).
Stalder, A.K. et al. Invasion of hematopoietic cells into the brain of amyloid precursor protein transgenic mice. J. Neurosci. 25, 11125–11132 (2005).
Massengale, M., Wagers, A.J., Vogel, H. & Weissman, I.L. Hematopoietic cells maintain hematopoietic fates upon entering the brain. J. Exp. Med. 201, 1579–1589 (2005).
van Furth, R. & Cohn, Z.A. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 128, 415–435 (1968).
Gordon, S. & Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 (2005).
Geissmann, F., Jung, S. & Littman, D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19, 71–82 (2003).
Grage-Griebenow, E., Flad, H.D. & Ernst, M. Heterogeneity of human peripheral blood monocyte subsets. J. Leukoc. Biol. 69, 11–20 (2001).
Sunderkotter, C. et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 172, 4410–4417 (2004).
Swirski, F.K. et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 117, 195–205 (2007).
Sedgwick, J.D. et al. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc. Natl. Acad. Sci. U.S.A 88, 7438–7442 (1991).
Serbina, N.V. & Pamer, E.G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7, 311–317 (2006).
Kuziel, W.A. et al. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Natl. Acad. Sci. U.S.A 94, 12053–12058 (1997).
Furuya, T. et al. Establishment of modified chimeric mice using GFP bone marrow as a model for neurological disorders. Neuroreport 14, 629–631 (2003).
Linard, C. et al. Acute induction of inflammatory cytokine expression after gamma-irradiation in the rat: effect of an NF-kappaB inhibitor. Int. J. Radiat. Oncol. Biol. Phys. 58, 427–434 (2004).
Francois, S. et al. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells 24, 1020–1029 (2006).
Kondo, A., Nakano, T. & Suzuki, K. Blood-brain barrier permeability to horseradish peroxidase in twitcher and cuprizone-intoxicated mice. Brain Res. 425, 186–190 (1987).
Bakker, D.A. & Ludwin, S.K. Blood-brain barrier permeability during Cuprizone-induced demyelination. Implications for the pathogenesis of immune-mediated demyelinating diseases. J. Neurol. Sci. 78, 125–137 (1987).
McMahon, E.J., Suzuki, K. & Matsushima, G.K. Peripheral macrophage recruitment in cuprizone-induced CNS demyelination despite an intact blood-brain barrier. J. Neuroimmunol. 130, 32–45 (2002).
Alliot, F., Godin, I. & Pessac, B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res. Dev. Brain Res. 117, 145–152 (1999).
Wegiel, J. et al. Reduced number and altered morphology of microglial cells in colony stimulating factor-1–deficient osteopetrotic op/op mice. Brain Res. 804, 135–139 (1998).
Beers, D.R. et al. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 103, 16021–16026 (2006).
Vallieres, L. & Sawchenko, P.E. Bone marrow–derived cells that populate the adult mouse brain preserve their hematopoietic identity. J. Neurosci. 23, 5197–5207 (2003).
Simard, A.R. & Rivest, S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. FASEB J. 18, 998–1000 (2004).
Biffi, A. et al. Correction of metachromatic leukodystrophy in the mouse model by transplantation of genetically modified hematopoietic stem cells. J. Clin. Invest. 113, 1118–1129 (2004).
El Khoury, J. et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 13, 432–438 (2007).
Remington, L.T., Babcock, A.A., Zehntner, S.P. & Owens, T. Microglial recruitment, activation and proliferation in response to primary demyelination. Am. J. Pathol. 170, 1713–1724 (2007).
Yamamoto, M. et al. Overexpression of monocyte chemotactic protein-1/CCL2 in beta-amyloid precursor protein transgenic mice show accelerated diffuse beta-amyloid deposition. Am. J. Pathol. 166, 1475–1485 (2005).
Prinz, M. et al. Positioning of follicular dendritic cells within the spleen controls prion neuroinvasion. Nature 425, 957–962 (2003).
Prinz, M. et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J. Clin. Invest. 116, 456–464 (2006).
Greter, M. et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med. 11, 328–334 (2005).
Okabe, M., Ikawa, M., Kominami, K., Nakanishi, T. & Nishimune, Y. 'Green mice' as a source of ubiquitous green cells. FEBS Lett. 407, 313–319 (1997).
Jung, S. et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Mack, M. et al. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J. Immunol. 166, 4697–4704 (2001).
Merkler, D. et al. Multicontrast MRI of remyelination in the central nervous system. NMR Biomed. 18, 395–403 (2005).
van Loo, G. et al. Inhibition of transcription factor NF-kappaB in the central nervous system ameliorates autoimmune encephalomyelitis in mice. Nat. Immunol. 7, 954–961 (2006).
Prinz, M. & Hanisch, U.K. Murine microglial cells produce and respond to interleukin-18. J. Neurochem. 72, 2215–2218 (1999).
Acknowledgements
We thank O. Kowatsch, M. Schedensack, E. Pralle, P. Grämmel and S. Blumenau for excellent technical assistance. We thank S. Jung (Rehovot, Israel) for scientific input. This work was supported by grants from Gemeinnützige Hertie-Stiftung to M.P. and D.M., Deutsche Forschungsgemeinschaft (SFB 507 A5 and PR 577) to J.P. and M.P. This project was supported by the DFG research Center for Molecular Physiology of the Brain Göttingen, but support was unfortunately discontinued. M.H. is supported by the Swiss MS Society and the Prof. Dr. Max-Cloëtta Foundation. A.M. and H.S. are fellows of the Gertrud Reemtsma Foundation.
Author information
Authors and Affiliations
Contributions
A.M., H.S., M.N., D.M., U.-K.H., J.P. and M.P. conducted the experiments. M.M., M.H. and W.B. provided reagents and scientific input. A.M., J.P. and M.P. designed the experiments and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2 (PDF 119 kb)
Rights and permissions
About this article
Cite this article
Mildner, A., Schmidt, H., Nitsche, M. et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci 10, 1544–1553 (2007). https://doi.org/10.1038/nn2015
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn2015
This article is cited by
-
The molecular determinants of microglial developmental dynamics
Nature Reviews Neuroscience (2024)
-
The niche matters: origin, function and fate of CNS-associated macrophages during health and disease
Acta Neuropathologica (2024)
-
Border-associated macrophages promote cerebral amyloid angiopathy and cognitive impairment through vascular oxidative stress
Molecular Neurodegeneration (2023)
-
TREM2hi resident macrophages protect the septic heart by maintaining cardiomyocyte homeostasis
Nature Metabolism (2023)
-
Mechanisms of myeloid cell entry to the healthy and diseased central nervous system
Nature Immunology (2023)