Abstract
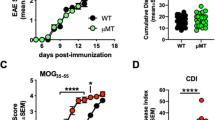
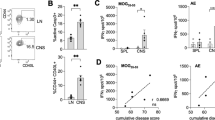
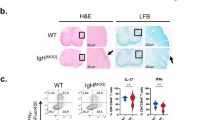
Inhibitors associated with CNS myelin are thought to be important in the failure of axons to regenerate after spinal cord injury and in other neurodegenerative disorders. Here we show that targeting the CNS-specific inhibitor of neurite outgrowth Nogo A by active immunization blunts clinical signs, demyelination and axonal damage associated with experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis (MS). Mice vaccinated against Nogo A produce Nogo-specific antibodies that block the neurite outgrowth inhibitory activity associated with CNS myelin in vitro. Passive immunization with anti-Nogo IgGs also suppresses EAE. Our results identify Nogo A as an important determinant of the development of EAE and suggest that its blockade may help to maintain and/or to restore the neuronal integrity of the CNS after autoimmune insult in diseases such as MS. Our finding that Nogo A is involved in CNS autoimmune demyelination indicates that this molecule may have a far more complex role than has been previously anticipated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Keegan, B.M. & Noseworthy, J.H. Multiple sclerosis. Annu. Rev. Med. 53, 285–302 (2002).
Steinman, L., Martin, R., Bernard, C.C., Conlon, P. & Oksenberg, J.R. Multiple sclerosis: deeper understanding of its pathogenesis reveals new targets for therapy. Annu. Rev. Neurosci. 25, 491–505 (2002).
Baranzini, S.E. et al. B cell repertoire diversity and clonal expansion in multiple sclerosis brain lesions. J. Immunol. 163, 5133–5144 (1999).
Berger, T. et al. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N. Engl. J. Med. 349, 139–145 (2003).
Robinson, W.H. et al. Protein microarrays guide tolerizing DNA vaccine treatment of autoimmune encephalomyelitis. Nat. Biotechnol. 21, 1033–1039 (2003).
Trapp, B.D., Ransohoff, R. & Rudick, R. Axonal pathology in multiple sclerosis: relationship to neurologic disability. Curr. Opin. Neurol. 12, 295–302 (1999).
Onuki, M., Ayers, M.M., Bernard, C.C. & Orian, J.M. Axonal degeneration is an early pathological feature in autoimmune-mediated demyelination in mice. Microsc. Res. Tech. 52, 731–739 (2001).
Schwab, M.E. & Caroni, P. Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J. Neurosci. 8, 2381–2393 (1988).
GrandPre, T., Nakamura, F., Vartanian, T. & Strittmatter, S.M. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature 403, 439–444 (2000).
Fournier, A.E., GrandPre, T. & Strittmatter, S.M. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 409, 341–346 (2001).
Wang, K.C., Kim, J.A., Sivasankaran, R., Segal, R. & He, Z. p75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature 420, 74–78 (2002).
Domeniconi, M. et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron 35, 283–290 (2002).
Wang, K.C. et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature 417, 941–944 (2002).
GrandPre, T., Li, S. & Strittmatter, S.M. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature 417, 547–551 (2002).
Hauben, E. et al. Vaccination with a Nogo-A-derived peptide after incomplete spinal-cord injury promotes recovery via a T-cell-mediated neuroprotective response: comparison with other myelin antigens. Proc. Natl. Acad. Sci. USA 98, 15173–15178 (2001).
Bernard, C.C. et al. Myelin oligodendrocyte glycoprotein: a novel candidate autoantigen in multiple sclerosis. J. Mol. Med. 75, 77–88 (1997).
Slavin, A. et al. Induction of a multiple sclerosis-like disease in mice with an immunodominant epitope of myelin oligodendrocyte glycoprotein. Autoimmunity 28, 109–120 (1998).
Zheng, B. et al. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron 38, 213–224 (2003).
Nicholson, L.B. & Kuchroo, V.K. Manipulation of the Th1/Th2 balance in autoimmune disease. Curr. Opin. Immunol. 8, 837–842 (1996).
Hauben, E. et al. Posttraumatic therapeutic vaccination with modified myelin self-antigen prevents complete paralysis while avoiding autoimmune disease. J. Clin. Invest. 108, 591–599 (2001).
Acevedo, L. et al. A new role for Nogo as a regulator of vascular remodeling. Nat. Med. 10, 382–388 (2004).
Merrill, J.L. & Benveniste, E.N. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci. 19, 331–338 (1996).
Marusic, S. & Tonegawa, S. Tolerance induction and autoimmune encephalomyelitis amelioration after administration of myelin basic protein–derived peptide. J. Exp. Med. 186, 507–515 (1997).
Bettelli, E. et al. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J. Immunol. 161, 3299–3306 (1998).
Merkler, D. et al. Rapid induction of autoantibodies against Nogo-A and MOG in the absence of an encephalitogenic T cell response: implication for immunotherapeutic approaches in neurological diseases. FASEB J. 17, 2275–2277 (2003).
Weiner, H.L. Oral tolerance: immune mechanisms and the generation of Th3-type TGF-β-secreting regulatory cells. Microbes Infect. 3, 947–954 (2001).
Bethea, J.R. et al. Systemically administered interleukin-10 reduces tumor necrosis factor-α production and significantly improves functional recovery following traumatic spinal cord injury in rats. J. Neurotrauma 16, 851–863 (1999).
Fawcett, J.W. & Asher, R.A. The glial scar and central nervous system repair. Brain Res. Bull. 49, 377–391 (1999).
Huber, A.B., Weinmann, O., Brosamle, C., Oertle, T. & Schwab, M.E. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J. Neurosci. 22, 3553–3567 (2002).
Ahmed, Z. et al. Myelin/axonal pathology in interleukin-12 induced serial relapses of experimental allergic encephalomyelitis in the Lewis rat. Am. J. Pathol. 158, 2127–2138 (2001).
Kawakami, N. et al. The activation status of neuroantigen-specific T cells in the target organ determines the clinical outcome of autoimmune encephalomyelitis. J. Exp. Med. 199, 185–197 (2004).
Becher, B., Durell, B.G., Miga, A.V., Hickey, W.F. & Noelle, R.J. The clinical course of experimental autoimmune encephalomyelitis and inflammation is controlled by the expression of CD40 within the central nervous system. J. Exp. Med. 193, 967–974 (2001).
Brosamle, C., Huber, A.B., Fiedler, M., Skerra, A. & Schwab, M.E. Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment. J. Neurosci. 20, 8061–8068 (2000).
Buffo, A. et al. Application of neutralizing antibodies against NI-35/250 myelin-associated neurite growth inhibitory proteins to the adult rat cerebellum induces sprouting of uninjured purkinje cell axons. J. Neurosci. 20, 2275–2286 (2000).
Linington, C., Engelhardt, B., Kapocs, G. & Lassman, H. Induction of persistently demyelinated lesions in the rat following the repeated adoptive transfer of encephalitogenic T cells and demyelinating antibody. J. Neuroimmunol. 40, 219–224 (1992).
Achiron, A. et al. Intravenous immunoglobulin treatment of experimental T cell–mediated autoimmune disease. Upregulation of T cell proliferation and downregulation of tumor necrosis factor α secretion. J. Clin. Invest. 93, 600–605 (1994).
Mistunaga, Y. et al. Direct evidence that a human antibody derived from patient serum can promote myelin repair in a mouse model of chronic-progressive demyelinating disease. FASEB J. 16, 1325–1327 (2002).
Miller, D.J., Bright, J.J., Sriram, S. & Rodriguez, M. Successful treatment of established relapsing experimental autoimmune encephalomyelitis in mice with a monoclonal natural autoantibody. J. Neuroimmunol. 75, 204–209 (1997).
Bandtlow, C., Zachleder, T. & Schwab, M.E. Oligodendrocytes arrest neurite growth by contact inhibition. J. Neurosci. 10, 3837–3848 (1990).
Okuda, Y., Okuda, M. & Bernard, C.C. The suppression of T cell apoptosis influences the severity of disease during the chronic phase but not the recovery from the acute phase of experimental autoimmune encephalomyelitis in mice. J. Neuroimmunol. 131, 115–125 (2002).
McQualter, J.L. et al. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J. Exp. Med. 194, 873–882 (2001).
Buffo, A., Carulli, D., Rossi, F. & Strata, P. Extrinsic regulation of injury/growth-related gene expression in the inferior olive of the adult rat. Eur. J. Neurosci. 18, 2146–2158 (2003).
Reindl, M. et al. Serum and cerebrospinal fluid antibodies to Nogo-A in patients with multiple sclerosis and acute neurological disorders. J. Neuroimmunol. 145, 139–147 (2003).
Karim, F., Volker, D. & Schwab, M.E. Improving axonal growth and functional recovery after experimental spinal cord injury by neutralizing myelin associated inhibitors. Brain Res. Rev. 36, 204–212 (2001).
Huang, D.W., McKerracher, L., Braun, P.E. & David, S. A therapeutic vaccine approach to stimulate axon regeneration in the adult mammalian spinal cord. Neuron 24, 639–647 (1999).
Sicotte, M. et al. Immunization with myelin or recombinant Nogo-66/MAG in alum promotes axon regeneration and sprouting after corticospinal tract lesions in the spinal cord. Mol. Cell. Neurosci. 23, 251–263 (2003).
Liu, J. et al. TNF is a potent anti-inflammatory cytokine in autoimmune-mediated demyelination. Nat. Med. 4, 78–83 (1998).
Barres, B.A., Silverstein, B.E., Corey, D.P. & Chun, L.L. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron 1, 791–803 (1988).
Meyer-Franke, A., Kaplan, M.R., Pfrieger, F.W. & Barres, B.A. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron 15, 805–819 (1995).
Norton, W.T. & Poduslo, S.E. Myelination in rat brain: method of myelin isolation. J. Neurochem. 21, 749–757 (1973).
Acknowledgements
We thank A. Slavin and S. Baranzini for suggestions and reviewing the manuscript; R. Sobel for help with histology; S. Dunn for the MBP-specific transgenic mice; L. Steinman and P. Fontoura for sharing unpublished results on Nogo A and L. Hazelwood and L. Lee for technical assistance. The work done in C.C.A.B.'s laboratory was supported by grants from the National Health and Medical Research Council of Australia and the US National Multiple Sclerosis Society, as well as a generous donation from Towards a Cure–MS, Australia. W.M. is a recipient of a US National Multiple Sclerosis Society Fellowship. B.Z. is a recipient of a Helen Hay Whitney Fellowship. B.B.'s laboratory is supported by a grant from the National Eye Institute (R01 EY10257) and M.T.-L.'s laboratory by a grant from the International Spinal Research trust. M.T.-L. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Karnezis, T., Mandemakers, W., McQualter, J. et al. The neurite outgrowth inhibitor Nogo A is involved in autoimmune-mediated demyelination. Nat Neurosci 7, 736–744 (2004). https://doi.org/10.1038/nn1261
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1261