Abstract
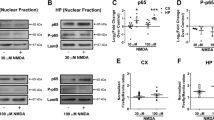
CA1 pyramidal neurons degenerate after transient forebrain ischemia, whereas neurons in other regions of the hippocampus remain intact. Here we show that in rat hippocampal CA1 neurons, forebrain ischemia induces the phosphorylation of the N-methyl-D-aspartate (NMDA) receptor 2A subunit at Ser1232 (phospho-Ser1232). Ser1232 phosphorylation is catalyzed by cyclin-dependent kinase 5 (Cdk5). Inhibiting endogenous Cdk5, or perturbing interactions between Cdk5 and NR2A subunits, abolished NR2A phosphorylation at Ser1232 and protected CA1 pyramidal neurons from ischemic insult. Thus, we conclude that modulation of NMDA receptors by Cdk5 is the primary intracellular event underlying the ischemic injury of CA1 pyramidal neurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Nakatomi, H. et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110, 429–441 (2002).
Jover, T. et al. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J. Neurosci. 22, 2115–2124 (2002).
Choi, D.W. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 11, 465–469 (1988).
Choi, D.W. Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci. 18, 58–60 (1995).
Simon, R.P., Swan, J.H., Griffiths, T. & Meldrum, B.S. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science 226, 850–852 (1984).
Faden, A.I., Demediuk, P., Panter, S.S. & Vink, R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science 244, 798–800 (1989).
Sattler, R. & Tymianski, M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol. Neurobiol. 24, 107–129 (2001).
Hollmann, M. & Heinemann, S. Cloned glutamate receptors. Annu. Rev. Neurosci. 17, 31–108 (1994).
Kutsuwada, T. et al. Molecular diversity of the NMDA receptor channel. Nature 358, 36–41 (1992).
Meguro, H. et al. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature 357, 70–704 (1992).
Monyer, H. et al. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science 256, 1217–1221 (1992).
Cull-Candy, S., Brickley, S. & Farrant, M. NMDA receptor subunits: diversity, development and disease. Curr. Opin. Neurobiol. 11, 327–335 (2001).
Abel, T. & Lattal, K.M. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr. Opin. Neurobiol. 11, 180–187 (2001).
Lee, J.M., Zipfel, G.J. & Choi, D.W. The changing landscape of ischaemic brain injury mechanisms. Nature 399 (Suppl.), A7–14 (1999).
Meldrum, B. & Garthwaite, J. Excitatory amino acid neurotoxicity and neurodegenerative diseases. Trends Pharmacol. Sci. 11, 379–387 (1990).
Scannevin, R.H. & Huganir, R.L. Postsynaptic organization and regulation of excitatory synapses. Nat. Rev. Neurosci. 1, 133–141 (2000).
Ali, D.W & Salter, M.W. NMDA receptor regulation by Src kinase signalling in excitatory synaptic transmission and plasticity. Curr. Opin. Neurobiol. 11, 336–342 (2001).
Li, B.S. et al. Regulation of NMDA receptors by cyclin-dependent kinase-5. Proc. Natl. Acad. Sci. USA 98, 12742–12747 (2001).
Lew, J., Beaudette, K., Litwin, C.M. & Wang, J.H. Purification and characterization of a novel proline-directed protein kinase from bovine brain. J. Biol. Chem. 267, 13383–13390 (1992).
Tsai, L.H., Delalle, I., Caviness, V.S., Chae, T. & Harlow, E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature 371, 419–423 (1994).
Greengard, P., Allen, P.B. & Nairn, A.C. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron 23, 435–447 (1999).
Chae, T. et al. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron 18, 29–42 (1997).
Patrick, G. et al. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402, 615–622 (1999).
Nguyen, M.D., Larivirre, RC. & Julien, J.P. Deregulation of Cdk5 in a mouse model of ALS: toxicity alleviated by perikaryal neurofilament inclusions. Neuron 30, 135–147 (2001).
Lee, M.S., Kwon, Y.T., Li, M., Peng, J., Friedlander, R.M. & Tsai, L.H. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405, 360–364 (2000).
Ohshima, T. et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl. Acad. Sci. USA 93, 11173–11178 (1996).
Nikolic, M., Dudek, H., Kwon, Y.T., Ramos, Y.F. & Tsai, L.H. The Cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 10, 816–825 (1996).
Barria, A. & Malinow, R. Subunit-specific NMDA receptor trafficking to synapses. Neuron 35, 345–353 (2002).
Carroll, R.C. & Zukin, R.S. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 25, 571–577 (2002).
Gong, X. et al. Cdk5-mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity-induced apoptosis. Neuron 38, 33–46 (2003).
Mitani, A. et al. Postischemic enhancements of N-methyl-D-aspartic acid (NMDA) and non-NMDA receptor-mediated responses in hippocampal CA1 pyramidal neurons. J. Cereb. Blood Flow Metab. 18, 1088–1098 (1998).
Andine, P., Jacobson, I. & Hagberg, H. Calcium uptake evoked by electrical stimulation is enhanced postischemically and precedes delayed neuronal death in CA1 of rat hippocampus: involvement of N-methyl-D-aspartate receptors. J. Cereb. Blood Flow. Metab. 8, 799–807 (1988).
Kirino, T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 239, 57–69 (1982).
Benveniste, H., Drejer, J., Schousboe, A. & Diemer, N.H. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J. Neurochem. 43, 1369–1374 (1984).
Hagberg, H. et al. Ischemia-induced shift of inhibitory and excitatory amino acids from intra- to extracellular compartments. J. Cereb. Blood Flow Metab. 5, 413–419 (1985).
Migaud, M. et al. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 396, 433–439 (1998).
Brenman, J.E. et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 84, 757–767 (1996).
Dawson, V.L., Dawson, T.M., London, E.D., Bredt, D.S. & Snyder, S.H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc. Natl. Acad. Sci. USA 88, 6368–6371 (1991).
Wang, F., O'Hare, M.J. & Park, D.S. Cyclin-dependent kinases and stroke. Expert. Opin. Ther. Targets 5, 557–567 (2001).
Neumar, R.W. et al. Calpain activity in the rat brain after transient forebrain ischemia. Exp. Neurol. 170, 27–35 (2001).
Liu, H.N., Giasson, B.I., Mushynski, W.E. & Almazan, G. AMPA receptor-mediated toxicity in oligodendrocyte progenitors involves for radical generation and activation of JNK, calpain and caspase 3. J. Neurochem. 82, 398–409 (2002).
Minger, S.L. et al. Glutamate receptor antagonists inhibit calpain-mediated cytoskeletal proteolysis in focal cerebral ischemia. Brain Res. 810, 181–199 (1998).
Jonas, P., & Burnashev, N. Molecular mechanisms controlling calcium entry through AMPA-type glutamate receptor channels. Neuron 15, 987–990 (1995).
Geiger, J.R. et al. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 15, 193–204 (1995).
Gorter, J.A. et al. Global ischemia induces downregulation of Glur2 mRNA and increases AMPA receptor-mediated Ca2+ influx in hippocampal CA1 neurons of gerbil. J. Neurosci. 17, 6179–6188 (1997).
Pellegrini-Giampietro, D.E., Gorter, J.A., Bennett, M.V & Zukin, R.S. The GluR2 (GluR-B) hypothesis: Ca2+-permeable AMPA receptors in neurological disorders. Trends Neurosci. 20, 464–470 (1997).
Zhu, D.Y., Liu, S.H., Sun, S. & Lu, Y.M. Expression of inducible nitric oxide synthase after focal cerebral ischemia stimulates neurogenesis in the adult rodent dentate gyrus. J. Neurosci. 23, 223–239 (2003).
Liu, S.H., Wang, J., Zhu, D.Y., Fu, Y.P. & Lu, Y.M. Generation of functional inhibitory neurons in the adult rat hippocampus. J. Neurosci. 23, 732–736 (2003).
Wang, J. et al. Interaction of calcineurin and type-A GABA receptor γ2 subunits produces long-term depression at CA1 inhibitory synapses. J. Neurosci. 23, 826–836 (2003).
Carlin, R.K., Grab, D.J., Cohen, R.S. & Siekevitz, P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J. Cell Biol. 86, 831–845 (1980).
Acknowledgements
This work was supported by grants (to Y.M.L.) from the Canadian Institute of Health Research, the Heart and Stroke Foundation (Canada), the Alberta Heritage Foundation for Medical Research, the Canada Foundation for Innovation and the Alberta Department of Innovation and Science. We thank K. Lukowiak for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wang, J., Liu, S., Fu, Y. et al. Cdk5 activation induces hippocampal CA1 cell death by directly phosphorylating NMDA receptors. Nat Neurosci 6, 1039–1047 (2003). https://doi.org/10.1038/nn1119
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1119